1. Background:
Spinal anesthesia is the anesthetic method of choice for cesarean delivery due to the fact that it is fast-onset and causes appropriate postoperative pain relief. It also has a lower risk of maternal death than general anesthesia (1, 2). Among the disadvantages of this technique, causing mothers to be less enthusiastic about it, are puncture pain, the patient’s fear of injection, and recall of the procedure. Anxiety at the beginning of the procedure is a natural reaction but causes pathophysiological responses in the body during surgery. According to studies, between 60% and 80% of individuals become anxious about the procedure, and many researchers believe that anxiety increases the need for high-dose analgesics after the operation, prolonging the duration of hospitalization. In those with a high level of anxiety, prolonged surgery in an inappropriate position and inadequate nerve block cause discomfort during surgery. These stressors highlight the importance of sedation during regional anesthesia techniques, including analgesia, anxiolysis, and amnesia (3, 4). During surgery, sedation requires the administration of a range of anti-anxiety and general anesthetic agents as a part of patient management for those undergoing regional anesthesia and remaining awake during surgery. The amnesia created contributes to surgical tolerance and helps the patient feel more comfortable and satisfied during the procedure (3).
Midazolam is a benzodiazepine-derived drug that effectively decreases anxiety without causing cardiorespiratory instability. Although this agent can create good sedation, ideal anterograde amnesia, satisfactory operation setting, and patient satisfaction, it is associated with delayed recovery and the return of psychomotor function and causes memory and respiratory disturbance (5). Today, α-2 agonists are increasingly being used in modern anesthesia due to several advantages such as effective sedation, analgesia, fewer arousal responses during surgery, and reduced need for anesthetic medications. In addition, it has been shown that the elevated concentration of catecholamines around surgery reduces adrenaline serum levels, thus stabilizing hemodynamic changes. These medications are also used for inducing regional anesthesia, increasing the duration of sensory and motor nerve block (6). In recent years, dexmedetomidine has been introduced as a newly developed drug and an active medetomidine isomer with high specificity and agonistic activity for α-2 receptors, promoting central sympathetic effects. It also has anti-anxiety, sedation, and analgesic effects with the least cardiovascular side effects, offering dexmedetomidine as one of the most suitable pre-operative medications (7-9). Dexmedetomidine is a lipophilic drug that easily passes through the blood-brain barrier and possesses 94% protein binding ability. This agent is widely used for inducing anesthesia in the ICU since it does not interfere with the function of other protein-binding medications. Dexmedetomidine has a fast-release time with a half-life of 6 minutes, an elimination half-life of 2 hours, a clearance rate of 39 L/hr, with its context-sensitive half-life varying from 4 to 10 minutes, and an infusion time from 250 minutes to 8 hours (10, 11). Intravenous dexmedetomidine has been used to increase the duration of analgesia during delivery, which helps mothers cope with anxiety and maintain their hemodynamic stability without worrying about undesirable effects on the fetus (12-14). This medication was approved by the US Food and Drug Administration in 1999 for human use (15).
Although spinal anesthesia is a safe choice for cesarean section (CS), some disadvantages, such as pain, anxiety, and agitation, may occur for mothers during the operation. Sedation is an important component of spinal anesthesia to control these complications (3).
2. Objectives
Since dexmedetomidine is not commonly used for sedation during CS, this study was designed to compare the sedation efficacy of midazolam and dexmedetomidine during spinal anesthesia and the postnatal period among parturients undergoing CS. We also compared the duration of anesthesia and hemodynamic changes during surgery between the two groups.
3. Methods
In this randomized, double-blinded clinical trial, sedation rate was considered as the primary outcome, and given that there was no similar study on this topic, a pilot study was conducted to determine the sample size. So, for each group, 35 subjects were enrolled. After the initial outcome was achieved, taking into account α = 0.05, power of 80%, and an acceptable difference, the final sample size was calculated. Ten individuals who participated in the pilot study were also included in the final experiment. Parturients were randomly divided into two equal groups of 35 people using Rand list online randomization software and entered into the study by the consecutive accessible sampling method. After obtaining approval from the Ethics Committee of the Research Deputy of Tabriz University of Medical Sciences and the Iranian Registry for Clinical Trials (IRCT2016111410765N12, https://irct.ir/trial/11182), the parturients willing to participate in the study, after receiving adequate explanations and signing informed consent, who were candidates for CS surgery were included according to our inclusion criteria and were randomly divided into two equal groups.
ASA Class II pregnant women who were candidates for CS surgery under spinal anesthesia in the Al-Zahra Hospital of Tabriz, Iran, were enrolled in the study. Parturients with ASA class III or above, those becoming hypotensive during pregnancy, women with preeclampsia, allergies to medications, and any contraindication for spinal anesthesia, as well as those who decided to quit the research, were not included. Also, parturients in whom spinal anesthesia failed and/or had block levels lower than the limit for spinal anesthesia were excluded. Moreover, the occurrence of any unexpected event, including hypotension, during pregnancy or CS was considered an exclusion criterion. For all parturients, the indication of CS, vital signs such as blood pressure, heart rate, and arterial oxygen saturation were recorded after entering the operating room. After recording vital signs, all parturients received 10 mL/kg ringer serum, and then spinal anesthesia was performed at the L3-L4 or L4-L5 intervertebral space in the seated position (i.e., the parturient sat on the bed; both feet were placed on a chair, and hips and knees were flexed). For this purpose, a 25-Gauge Quincke spinal needle (Dr.Japan Co.,Ltd.) was used to infuse 2.5 mL Bupivacaine 0.5% (BUPIVACAINE MYLAN, 5 mg/mL) and Fentanyl (10 micrograms) to maintain an adequate level of anesthesia at T4-T5 levels during cesarean delivery. After anesthesia, the mothers were placed in a supine position, and the uterus was shifted toward the left by 15 degrees; then, 5 mL/min of oxygen was provided through a face mask for the mother. The level of anesthesia was monitored by the pinprick method prior to skin incision. Pinprick testing was conducted with a non-trauma tip needle and compared to a non-anesthetized part of the body (e.g., arm) so the patient could notice the difference. Blood pressure was evaluated by a non-invasive Blood Pressure (NIBP) monitor every 2 minutes until the umbilical cord was clamped and then every 5 minutes. The heart rate was evaluated continuously by ECG monitoring. The degree of motor block after spinal anesthesia was evaluated using a modified Bromage scale from the time of spinal anesthesia until the complete loss of anesthesia in PACU. The modified Bromage scale is a scoring system used to evaluate the degree of motor block after local anesthesia in a range from 0 to 3 (0 = ability to move the thigh, knees, and ankles, 1 = inability to move the thigh but able to move knees and ankles, 2 = unable to move the thigh and knee but able to move ankles, and 3 = inability to move the thigh, knees, and ankles). Hypotension was considered a reduction of more than 20% in the baseline blood pressure, which was treated with infusing 2.5 mg ephedrine intravenously.
All surgeries were performed by the same surgeon in the two groups. After delivery, in group M, 0.03 mg/kg of Midazolam (Midazolam [chemidarou], 5 mg/mL Amp) was injected intravenously. Parturients in group D received the same amount of placebo and then 1 μg/kg dexmedetomidine (Hospira, Inc. Lake Forest, IL 60045 USA ®Precedex) diluted in 20 mL of normal saline, which was infused immediately after the delivery during a period of 10 minutes. Parturients in group M were also infused with the same amount of normal saline. A trained individual was in charge of preparing and encoding the medications, midazolam, and normal saline in syringes of equal volumes, as well as 1 μg/kg of dexmedetomidine diluted in 20 mL of normal saline. The same volume of normal saline was prepared in another syringe. The person administering the medication and the patient assessor did not know the content of the syringes. Visual pain scoring (VAS) was used to evaluate the parturient’s pain severity before and during the surgery, as well as in the postoperative period up to 8 hours, by self-description from 0 = no pain to 10 = severe pain (16, 17). In those with a pain score of 4 ≤, 0.5-1 mg/kg meperidine was administered, and the dosage was recorded in a checklist. The duration of analgesia was considered from the time of spinal anesthesia until the patient was given a painkiller. The sedation level was evaluated and recorded during surgery and in the postoperative period based on the Ramsay sedation score from 1 = completely awake to 6 = failure to respond to painful stimuli from the time of the administration of midazolam or dexmedetomidine until the complete loss of analgesia. Meanwhile, the APGAR scores of the babies were also recorded in the first and fifth minutes. The occurrence of any side effects was monitored closely, such as nausea, vomiting, bradycardia, and hypotension (defined as a fall of more than 20% in BP and HR).
All data were recorded in a checklist, and finally, the data obtained were analyzed using descriptive statistics (mean ± SD and frequency), the RMA test, ratios’ comparison test, and mean difference test for independent groups. Analyses were conducted in SPSS 18 software, and a P value less than 0.05 was defined as statistically significant. Also, intention-to-treat and per-protocol analyses were performed. Meanwhile, relative risk (RR) with a 95% confidence interval was reported.
4. Results
This study was conducted on 70 pregnant women with ASA II who underwent CS with spinal anesthesia. The subjects were randomly divided into two groups of 35 parturients to receive either intravenous midazolam or intravenous dexmedetomidine. None of the mothers were excluded from the study. Table 1 shows the demographic data of the parturients in the two study groups, revealing no significant differences in age, height, and weight (P > 0.05).
| Variables | Midazolam | Dexmedetomidine | P-Value |
|---|---|---|---|
| Age (y) | 30.31 ± 4.83 | 30.54 ± 5.80 | 0.858 |
| Height (cm) | 161.09 ± 5.49 | 163.33 ± 5.33 | 0.086 |
| Weight (kg) | 74.77 ± 11.6 | 75.43 ± 10.34 | 0.803 |
Demographic Data of Participants
Table 2 demonstrates the indications of CS in both groups, showing no significant difference between the two study groups in this regard (P > 0.05).
| Cesarean Section Indications | Midazolam | Dexmedetomidine | P-Value |
|---|---|---|---|
| Repeated cesarean section | 20 (57.1) | 20 (57.1) | > 0.05 |
| Breech | 2 (5.7) | 1 (2.9) | |
| Lack of response to induction | 2 (5.7) | 4 (11.4) | |
| Pelvic stenosis | 3 (8.3) | 5 (14.3) | |
| Fetal bradycardia | 4 (11.4) | 4 (11.4) | |
| Descent arrest | 4 (11.4) | 1 (2.9) |
The Indications of Cesarean Section a
The comparison of the level of anesthesia (according to Bromage scoring system (1-3)) between the two groups showed that in group M, no patient had a score of 1, 16 (45.7%) had a score of 2, and 19 (54.3%) had a score of 3. In group D, 2 (5.7%), 17 (48.6%), and 16 (45.7%) subjects had scores of 1, 2, and 3, respectively. Comparison of the two groups did not show a significant difference in the levels of anesthesia (i.e., 1 to 3) between the two groups, with respective P values of 0.310, 0.210, and 0.260.
The two groups were compared in terms of their hemodynamic status after medication administration (Table 3). The comparison of blood pressure showed no significant difference between the two groups. The heart rate showed a significant reduction after the injection of dexmedetomidine (P = 0.025).
| Hemodynamic Parameters | Midazolam | Dexmedetomidine | P-Value |
|---|---|---|---|
| Systolic blood pressure | 107.74 ± 13.7 | 110.34 ± 13.19 | 0.170 |
| Diastolic blood pressure | 59.00 ± 12.6 | 63.94 ± 17.3 | 0.420 |
| Mean arterial pressure | 75.49 ± 11.8 | 78.69 ± 11.9 | 0.260 |
| Mean heart rate | 92.14 ± 16.02 | 83.43 ± 15.8 | 0.025 b |
Hemodynamic Status Changes After Medication Administration in the Two Study Groups
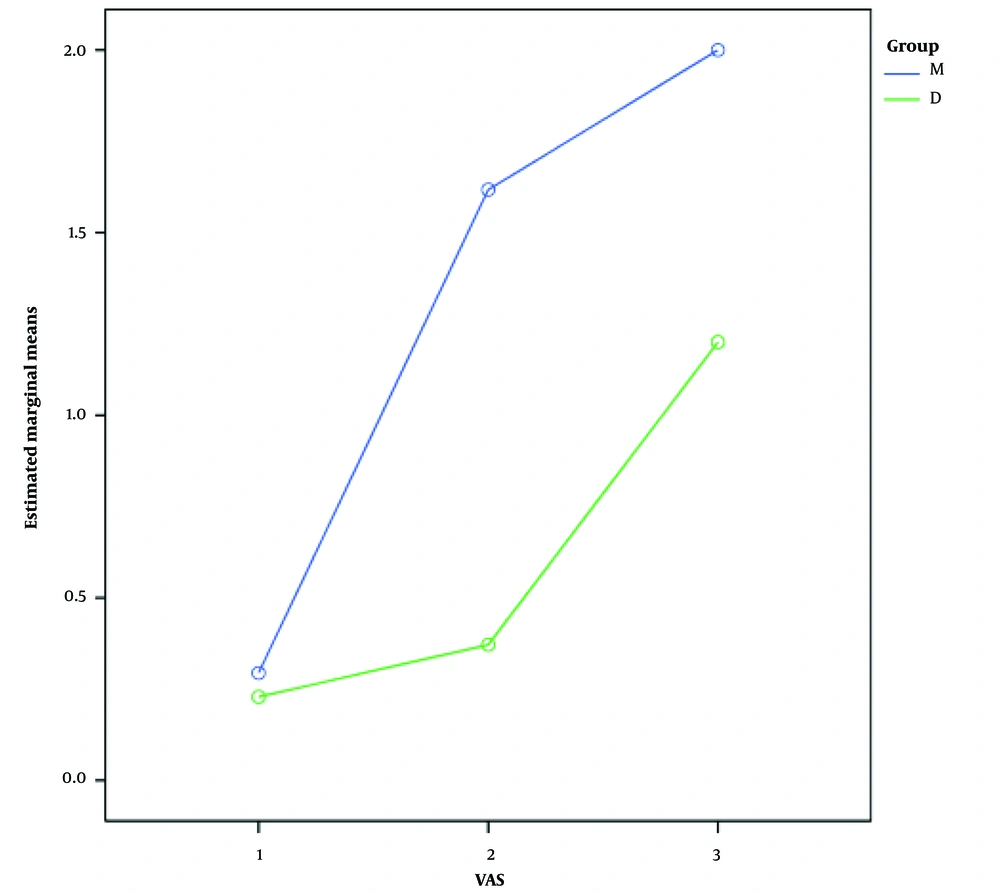
The severity of pain (based on VAS) during and after surgery was also compared between the two groups. The mean pain severity score in group M was 0.29 ± 0.6 during the operation, 1.6 ± 0.9 at the time of PACU entry, and 2 ± 0.69 at the PACU exit time. The mean pain severity score in group D was 0.23 ± 0.5 during the operation, 0.37 ± 0.8 at the time of PACU entry, and 1.2 ± 1.3 at the PACU exit time. Comparison of the pain severity score at different times showed an increasing trend in pain severity in both groups (P < 0.001); however, this score was significantly lower in the group receiving dexmedetomidine than in the group treated with midazolam (P < 0.001). Figure 1 compares pain severity during and after CS between the two study groups.
The interval between PACU entry and the first need for painkillers (i.e., a VAS score > 4) was evaluated in the two groups. In group M, the maximum time was 640 minutes, and the minimum time was equal to 120 minutes (with a median of 240 minutes). In group D, the maximum time was 720 minutes, and the minimum time was equal to 120 minutes (with a median of 360 minutes). Data analysis showed that the time to the first rescue analgesia was significantly longer in the dexmedetomidine group compared to the midazolam group (P < 0.001). Furthermore, the entire analgesic dose injected in the postoperative phase was 1467.5 ± 43.14 mg in the midazolam group and 977.5 ± 27.07 mg in the dexmedetomidine group, showing a significantly lower dose in the latter group (P < 0.001).
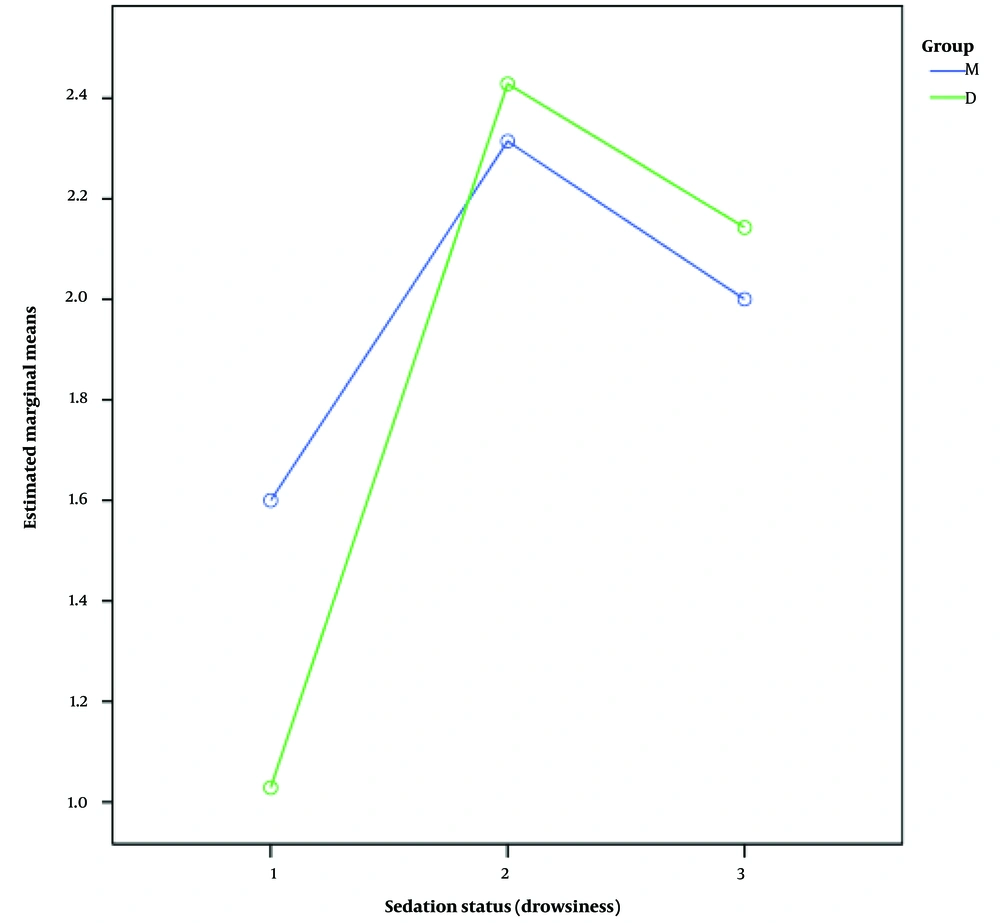
The means ± standard deviations of sedation status at different stages after anesthesia, after medication injection, and during recovery were 1.6 ± 0.6, 2.31 ± 0.83, and ± 2 ± 0.59 in group M, and 1.3 ± 0.92, 2.4 ± 0.84, and 2.1 ± 0.77 in group D, respectively. A comparison of drowsiness between the two groups showed no significant difference (P = 0.410). Figure 2 shows the status of sedation (drowsiness) in the two groups.
The two groups were also monitored for the incidence of any possible complications. In group M, only 5 (14.7%) parturients had hypotension, while no complication was observed in the rest of them (85.3%). In group D, 1 patient (2.9%) had hypotension; 1 patient (2.9%) had nausea; 4 patients (11.4%) developed transient bradycardia, and the remaining (82.9%) showed no problems. The comparison of the two groups showed no significant difference in terms of side effects (P = 0.054).
5. Discussion
The present study was conducted on 70 pregnant women who underwent SC under spinal anesthesia. The participants were divided into two groups of 35 people who received either intravenous midazolam (group M) or intravenous dexmedetomidine (group D). Our results indicated that dexmedetomidine administration caused more effective analgesia during and after surgery and a prolonged period of sensory block accompanied by proper sedation during the operation without any significant complication for parturients undergoing CS with spinal anesthesia. In our study, we investigated anesthesia level, which was classified according to the Bromage scoring system with analgesia scores of 1 to 3 (1 = not satisfactory, 2 = moderate, and 3 = satisfactory) between the two groups. The two groups showed no significant difference at any of the levels of anesthesia (1 to 3). In a study performed by Kaya et al., comparing the effects of dexmedetomidine and midazolam during spinal anesthesia, the mean level of anesthesia was reported to be 3 in all groups, with a range of 2 to 3 (18), which was consistent with the results of our study.
In the present study, pain severity during and after surgery was also compared between the two groups. The comparison of pain severity between the two groups showed that pain severity during surgery and in the postoperative recovery period was significantly lower in the dexmedetomidine group than in the midazolam group. A study by Kaya et al. on the analgesic effects of dexmedetomidine and midazolam during spinal anesthesia showed that postoperative pain was significantly lower in the parturients receiving dexmedetomidine (VAS = 2.1 ± 0.6) than those receiving midazolam (VAS = 2.8 ± 0.5) (18), which was consistent with our study. Tsaroucha et al. studied the analgesic efficacy of intrathecally administered fentanyl (F) compared to dexmedetomidine (D) during SC and found that parturients in group D had a longer duration of block than those in group F. Cardiovascular stability, neonatal Apgar scores, need for rescue analgesia, time to the first rescue analgesia, and maternal satisfaction with anesthesia/analgesia did not show statistically significant differences between the two groups (19). The results of different studies have also indicated that during anesthesia with bupivacaine, dexmedetomidine performed significantly better than midazolam in reducing postoperative pain (6, 7, 9).
In our study, the interval between the PACU entry time and the first need for pain relief based on the VAS standard (i.e., VAS > 4) was investigated in the two groups, showing that this period was significantly longer in the dexmedetomidine group than in the midazolam group. Furthermore, the total dose of the analgesic injected during the postoperative phase was significantly lower in the D group than in the M group. Consistent with our study, a study by Kaya et al. (18) on the effects of dexmedetomidine in comparison with midazolam during spinal anesthesia showed that the interval from PACU exit time till the first need for pain relief was significantly longer in the dexmedetomidine group (216 ± 43 min) than in the midazolam (136 ± 25 min) group. Also, in our study, the duration until the need for the first pain relief was more prolonged compared to the recent study, which may be due to the higher doses of the medications used in our study compared to theirs (18).
In our study, the duration of anesthesia was significantly longer in the parturients receiving dexmedetomidine than in those treated with midazolam. In a clinical review, alpha-2 agonists such as clonidine and dexmedetomidine were applied as adjuvants for inducing anesthesia (20, 21). It has also been reported that alpha-2 agonists can be used to induce regional anesthesia by influencing vasoconstriction and augmenting the block of C fibers and the flow of axonal retrograde in spinal nerves (22, 23).
In our study, sedation (drowsiness) at different post-anesthesia stages, after medication injection, and during recovery was compared between the 2 groups, revealing no significant difference in this regard between the study groups. Dinesh et al., in a study on 100 parturients, investigated the effects of intravenous dexmedetomidine on bupivacaine-induced spinal anesthesia. The dosage of dexmedetomidine was 1 μg/kg as an initial dose and 0.5 μg/kg/h afterward. In contrast to our results, the parturients receiving dexmedetomidine in the recent study showed a higher sedation rate (Ramsay score = 4.4 ± 0.7) than those receiving midazolam (Ramsay score = 2 ± 0.1) (24).
Davis et al. evaluated the effects of intravenous dexmedetomidine along with neuraxial anesthesia in mothers undergoing cesarean delivery. The primary outcome was the need for general anesthesia, and in contrast to our study, they observed the same rate of change to general anesthesia in the parturients who received dexmedetomidine compared to those receiving other drugs (25).
In our study, we noticed no significant difference between the two groups (i.e., midazolam and dexmedetomidine) in terms of the hemodynamic status (systolic, diastolic, and mean blood pressure). However, the heart rate was observed to be significantly lower after administrating dexmedetomidine compared to midazolam. Despite the association of dexmedetomidine with hypotension, restricting its maximal anesthetic dose, the prevalence of this complication is generally low due to the fact that the patient continues to receive hydration at different stages of operation and anesthesia (8). In a study conducted by Kaya et al. to investigate the effects of dexmedetomidine in comparison with midazolam during spinal anesthesia, as in our study, no significant differences were observed regarding mean arterial pressure and heart rate changes (18). In another study by Elcicek et al., bradycardia was detected in 30% of the recipients of dexmedetomidine. In our study, bradycardia was observed in 17% of the mothers receiving dexmedetomidine (8). In other studies, the prevalence of bradycardia after spinal anesthesia has been reported from 10% to 15%. Also, in a study conducted by Bloor et al., the rate of dexmedetomidine-induced bradycardia was reported as 25% (26). In another study, Dinesh et al. reported bradycardia in 33% of the patients treated with dexmedetomidine (24). The same as our study, Davis et al. demonstrated a statistically significant association between bradycardia occurrence and dexmedetomidine treatment, but no such association was seen for hypotension (25).
5.1. Conclusions
According to our results, it can be concluded that the use of intravenous dexmedetomidine compared with midazolam can increase the duration of spinal anesthesia due to deeper and more prolonged analgesia during and after the operation in the parturients undergoing CS with spinal anesthesia.