1. Introduction
During the 1980’s, mammography (MG) was established as the principal screening tool for breast cancer. However, its frequent application had some major consequences. On the one hand, there were differences in the technical parameters of examination (eg, intensity of radiation). On the other hand, the examination reports included different terms and definitions for describing the MG findings, lacked standardization, and were often merely descriptive, leading to a controversial clinical diagnosis and ultimately, lack of a clear follow-up or management plan (1). This concern was addressed by the American College of Radiology (ACR); accordingly, a qualification program for MG was developed in 1986.
Additionally, the ACR gathered a task force, including various medical associations and organizations (ie, the American Medical Association, National Cancer Institute, Centers for Disease Control and Prevention, Food and Drug Administration, American College of Surgeons, and College of American Pathologists) to establish relevant guidelines to ensure accurate evaluation and description of MG findings and provide a common communication tool for different medical specialties; this project was called the “Breast Imaging-Reporting and Data System” (BI-RADS). The first edition of BI-RADS was issued in 1993 and only involved MG. The subsequent editions were released in 1995, 1998, and 2003, and more recently, in 2013. In each edition, some modifications were made based on the users’ experiences. The fourth edition expanded the use of BI-RADS to breast ultrasound (US) and breast magnetic resonance imaging (MRI) (1). Moreover, the BI-RADS may be further improved to involve other newer imaging technologies, such as contrast-enhanced MG, positron emission MG, and similar modalities (2).
The clinical significance of the BI-RADS system in clinical practice is that it allows for a standardized communication of information about the breast imaging findings, both among radiologists and between radiologists and clinicians. More importantly, it can help physicians who are not specialized in breast imaging to identify the appropriate approach and management for the patient. Besides, one of the major advantages of BI-RADS is its comprehensiveness. In this study, we will first investigate the general structure of the BI-RADS and then assess each topic for MG, US, and MRI. Finally, the whole concept of the BI-RADS will be presented concisely and then summarized in a small table to make it as easy as possible.
2. General Report Structure
For optimal standardization, the BI-RADS first describes how an imaging report should be structured. Although it now involves three different imaging modalities, the essential details of MG, US, and MRI reports generally have a similar structure (3), which is as follows:
(1) The indication for examination is reported to determine if it is performed as a regular screening test, further investigation of a new lesion, or follow-up of a known lesion.
(2) The device model and technical parameters used for the examination are included in the MRI and US reports.
(3) A brief description of the overall composition of the breast is presented, depending on the type of examination (breast density in MG, type of echogenicity in US, and background parenchymal enhancement and extent of fibroglandular tissue in T1-weighted MRI).
(4) A thorough and detailed description of any imaging finding is presented.
(5) It is very important to compare the examination results with previous studies of the same type or with other types of investigations and also to study similar or stable imaging findings versus changes in imaging findings.
(6) Composite reports are an issue that have been only defined in the structure of ultrasound BI-RADS reports. When several imaging modalities have been applied, they need to be reported in different paragraphs of one report sheet, and the overall assessment and recommendations should be based on all the findings.
(7) In the next critical step, a radiologist presents an overall assessment of the findings and clearly states the BI-RADS category of the image.
(8) In the final step, a management suggestion is made based on the BI-RADS category.
3. Indications for Examinations
3.1. MG
In this type of examination, any of the various indications, including breast cancer screening in asymptomatic women, further evaluation of a clinical finding or recall for a finding on screening MG, follow-up in breast cancer survivors, or interval assessment of a known probably benign lesion, should be considered. If MG is requested to evaluate a finding, its characteristics and location should be documented in the report (4).
3.2. Ultrasound
The indications for ultrasound can be a complementary assessment of MRI- or MG-suspected lesions or a dense MG image, especially in women who are at an increased risk of breast cancer, and evaluation of a palpable lump; it is also applied as the first breast imaging modality for women with contraindications for MG (eg, young, pregnant, and lactating patients) (5).
3.3. MRI
The indications for MRI include breast cancer screening in high-risk groups; further evaluation of a new cancer for ruling out the presence of multicentric, multifocal, or bilateral tumors; interval assessment of a previously MRI-detected, probably benign lesion; evaluation of breast cancer before and after neoadjuvant chemotherapy; or assessment of equivocal or discordant findings on MG and US. A short history of the patient is preferred to be cited in this part of the report, including the patient’s menstrual or menopausal status, clinical findings, previous breast biopsy, and history of breast cancer treatment (6).
4. Examination Technique
4.1. MG
This topic has not been defined in MG reports; however, radiation dose and type of device may be considered important.
4.2. Ultrasound
This topic in the BI-RADS is called “statement of scope and technique” for breast US. The “scope” refers to the range of examination (whole breast or targeted to a particular lesion detected using MG or MRI), and the “technique” includes different options, such as color Doppler or shear wave elastography (5).
4.3. MRI
The technical details include pulse sequences (T1- and T2-weighted images with or without fat saturation), orientation, plane (axial, coronal, and sagittal), contrast injection, contrast dose, time of imaging relative to contrast injection, and performance of subtraction and maximal intensity projection (MIP) images (6).
5. A Succinct Description of the Overall Breast Composition
5.1. MG
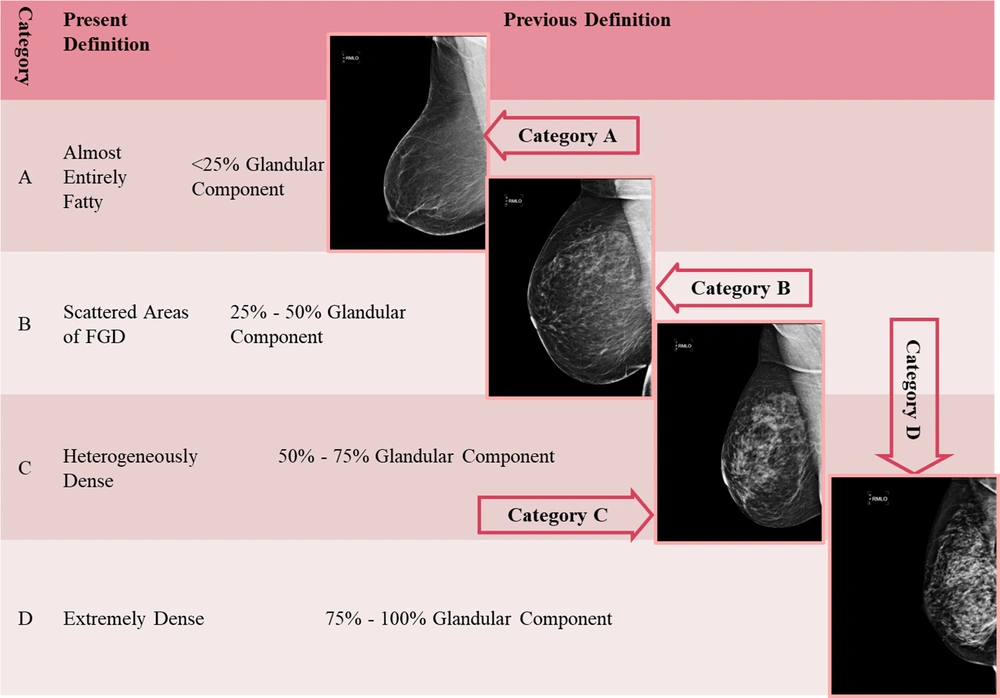
The density of the breast should be always reported based on the ACR classification, which includes four categories, as shown in Figure 1. The overall breast composition is a general estimate of the amount of fibroglandular tissue, which creates dense (or white) areas in an image relative to the fatty tissue that creates low-density (black) areas (Figure 1). As the percentage of dense areas increases, the possibility of an obscure breast mass is increased (4). Practically, this classification is of great clinical importance. The MG-based breast density has been shown to be a significant risk factor for breast cancer, and the relationship between various modifiable and non-modifiable factors and breast cancer has been investigated in recent years (7-9). Interestingly, no significant association has been found between the density of breast tissue in the physical breast examination and MG density (10). The false negative value of a dense MG finding may be as high as 20%. In this case, clinicians can evaluate how reliable and sensitive a given MG finding is and can request further examinations if needed (commonly a breast US examination).
5.2. Ultrasound
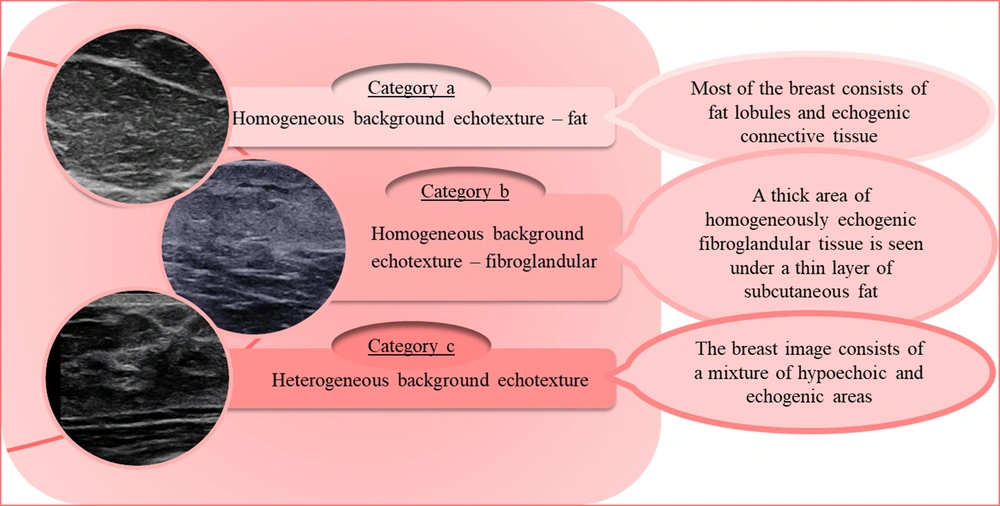
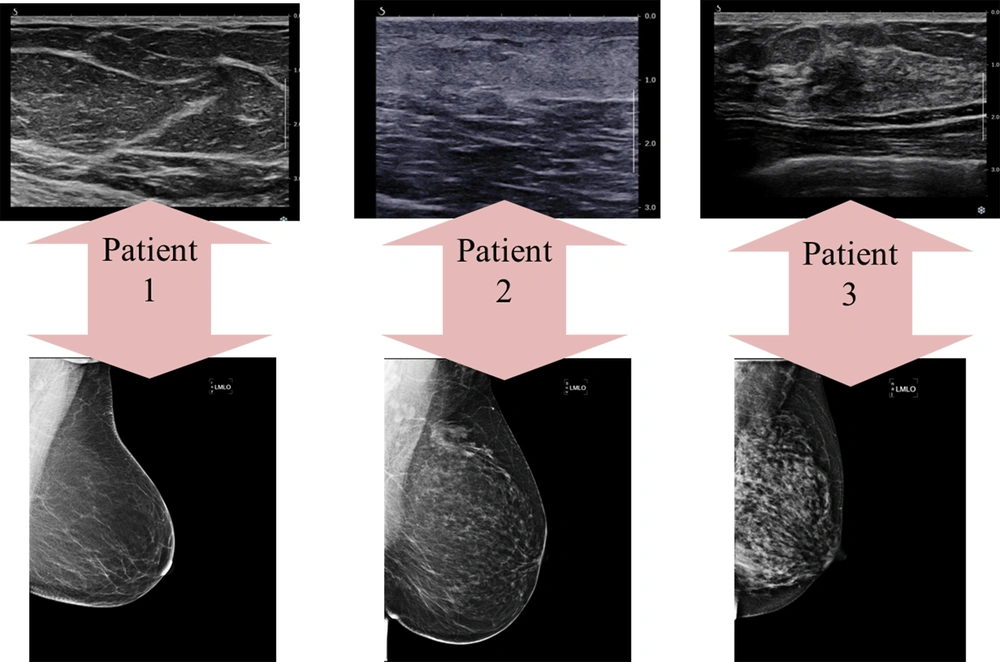
The breast tissue composition can be described via ultrasound using echogenicity rather than density. The parenchymal echogenicity is compared with that of subcutaneous fat. The tissue composition is classified into three types, which roughly resemble the four density categories in MG, as shown in Figure 2 (5). Regarding the breast density in MG, description of the breast echogenicity in ultrasound can significantly help us determine how reliable and sensitive a given ultrasound examination is. In other words, report of a heterogeneous background echotexture implies a probable interference with the detection of subtle lesions; this category is more frequently seen in young women (11). The breast tissue composition in MG and ultrasound images of three patients is demonstrated in Figure 3. As seen in this figure, the densities of the breast US and MG in one patient do not always correlate and might be completely different.
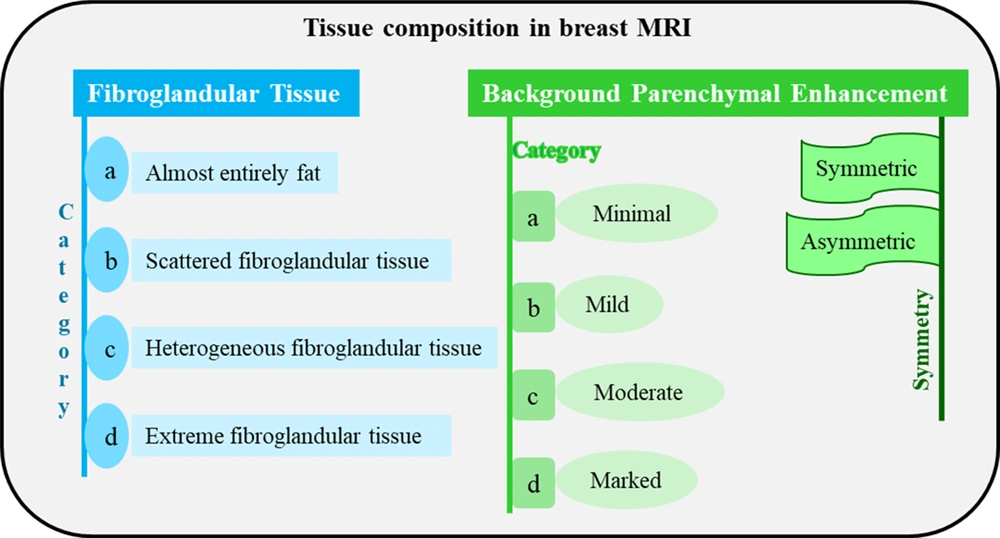
5.3. MRI
Three factors should be defined when reporting the category of breast tissue composition in MRI:
(1) Mass of the breast fibroglandular tissue according to T1-weighted images: It reflects normal enhancement of fibroglandular tissue and its progression over time; it is evaluated in the first series of post-contrast images. The amount of fibroglandular tissue in MRI is consistent with the MG description of breast density.
(2) Volume and intensity of background parenchymal enhancement (BPE) as detected visually: Of the three classic breast imaging examinations, breast MRI is the only one that uses a gadolinium-based contrast agent, at least in its conventional form. Therefore, this type of examination is largely based on tissue enhancement caused by the contrast agent. The BPE is described depending on the level of enhancement (minimal, mild, moderate, or marked) and symmetry/asymmetry. Moderate and marked levels of enhancement may obscure subtle suspicious findings in terms of carcinoma (either invasive carcinoma or in situ carcinoma) and decrease the MRI ability to find subtle tumors. An asymmetric BPE may be attributed to previous treatments, such as radiation therapy. There is no significant correlation between the amount of fibroglandular tissue and BPE in MRI.
(3) Presence of implants: If a patient has an implant in a breast(s), its content and number of lumens need to be determined. The tissue composition is defined bilaterally; therefore, when the fibroglandular tissue and BPE are not visually alike, the overall description is centered on the breast with larger amounts of fibroglandular tissue and BPE. Due to the lack of strong evidence, no categories are presented in percentage or quartile for them; instead, a specific terminology, as presented in Figure 4, can be used. Asymmetry and alterations due to previous treatments may be documented in this part of the BI-RADS report (6).
6. Clear Description of All Important Findings
6.1. MG
The BI-RADS has a structured system for description of MG findings, which is as follows (4):
(1) Masses: A mass is defined as a three-dimensional, space-occupying lesion that is seen in two different MG views. It has completely or partially convex borders. In the MG report, the mass descriptors are as follows:
• Shape: Oval (elliptical or egg-shaped), round (spherical or ball-shaped), and irregular (neither round nor oval).
• Margin: Circumscribed (well defined, at least 75% of the margin is sharply defined), obscured (mainly circumscribed, > 25% of the margin is hidden), microlobulated (with gentle lobulation), indistinct (with no clear demarcation of the entire lesion or parts of it relative to the surrounding tissue), or spiculated (with radiating lines in the margin).
• Density: It is used to define X-ray attenuation of the mass relative to the fibroglandular tissue. It is classified as high, equal, low, or fat-containing.
Among these characteristics, irregular shape, indistinct, microlobulated, or spiculated margins, and high mass density have high positive prognostic values for malignancy.
(2) Calcifications: Calcifications that are considered benign in MG are mainly larger, coarser, and round with smooth margins. They are mostly seen easily, with no need for magnification views in contrast to malignant calcifications that are usually very small and often require magnification for appropriate visualization. Every calcification should be assessed in terms of morphology and distribution.
• Morphology: It is classified as typically benign (round, vascular, coarse or popcorn-like, large rod-like, rim-like, and dystrophic calcifications, skin calcifications, old sutures, and “milk of calcium”) and suspicious (amorphous, coarse heterogeneous, fine pleomorphic, fine linear, or fine linear branching) calcifications.
• Distribution: It is described as diffuse, regional, grouped, linear, or segmental. A segmental distribution along one or several ducts raises the risk of malignancy and increases the level of suspicion for round and amorphous calcifications.
The presence of calcifications with a suspicious morphology on MG is classified as at least BI-RADS category 4 (see below, under “BI-RADS classification”), especially when the distribution is not diffuse.
(3) Architectural distortions: This term is used when thin straight lines or spiculations are radiating from a point. When there is no history of trauma or surgery, this can raise the suspicion of malignancy or a radial scar, and biopsy is warranted.
(4) Asymmetries: They are described as asymmetries between the two breasts. They do not meet the criteria of a mass, have concave margins, and are usually interspersed within fat. Asymmetries have four types:
• Asymmetry: An area of tissue with fibroglandular density that is visible in only one MG view.
• Focal asymmetry: An area of tissue with fibroglandular density that is seen in both views of one breast (mediolateral oblique and craniocaudal views) and involves less than one quadrant of the breast.
• Global asymmetry: A large fibroglandular density that occupies a volume of at least one quadrant of the breast in one view.
• Developing asymmetry: A focal asymmetry that is new, larger, or more conspicuous than the one observed in the previous MG image.
A developing asymmetry has a 15% risk of malignancy and should be biopsied unless a typical benign finding (eg, a simple cyst) is found in other complementary images. On the other hand, a global asymmetry is mostly a normal variant unless it is in association with an architectural distortion, a mass, or a suspicious calcification.
(5) Intramammary lymph nodes: Circumscribed masses with a bean-shaped morphology and hilar fat. They are generally less than 1 cm and constitute a benign finding.
(6) Skin lesions: A lesion over the skin in two MG views, mostly surrounded by an area of translucency; it is considered benign.
(7) Solitary dilated duct: A unilateral, tubular, or branching structure that can be a sign of non-calcified ductal carcinoma in situ (DCIS).
The associated features are findings that can be described along with any of the abovementioned lesions. They include skin retraction, nipple retraction, skin thickening, trabecular thickening, axillary lymphadenopathy, and even architectural distortions or calcifications when they are not dominant findings and accompany the main lesion. The location of lesion found in MG should be described according to four factors: (1) laterality: Right or left breast; (2) quadrant: Upper outer quadrant, upper inner quadrant, lower outer quadrant, lower inner quadrant, upper central (12 o’clock in a supposed clock face configuration), lower central (6 o’clock), central (directly behind the nipple areolar complex), retroareolar (central location in the anterior third), and axillary tail (upper outer quadrant near the axilla); (3) depth: Anterior, middle, and posterior third; and 4) distance from the nipple (in centimeters).
6.2. Ultrasound
Various findings are reported in an ultrasound (5):
(1) Mass: A mass is a three-dimensional space-occupying lesion. One of the advantages of ultrasound over MG is its capacity to define masses depending on their cystic or solid nature. The features of a mass are as follows:
• Shape: Oval (elliptical or egg-shaped), round (spherical or ball-shaped), or irregular (neither oval, nor round). The irregular margin should raise concerns of malignancy.
• Orientation: Parallel or unparallel relative to the skin line.
• Margin: Circumscribed (well-defined) or non-circumscribed (indistinct, angular, microlobulated, or spiculated).
• Echo pattern: It is defined in relation to fat and can be anechoic, hypoechoic, isoechoic, hyperechoic, or heteroechoic.
• Posterior features: They include enhancement, shadowing, combined pattern, or lack of posterior features. Posterior enhancement is mostly observed in cysts and benign solid masses; however, high-grade carcinoma may also show posterior enhancement. Shadowing is mostly related to fibrosis, with or without underlying carcinoma.
• Vascularity: Although it is described as an “associated feature” in the BI-RADS lexicon, this characteristic of a mass is assessed by color Doppler ultrasound and is classified as internal vascularity, vessels in rim, or no vascularity (absent).
• Elasticity: It is also defined as an “associated feature” in the BI-RADS lexicon. It describes a mass as soft, intermediate, or hard according to elastography.
(2) Calcifications: Although they are not as easily observed in ultrasound as in MG, improvements in the technology of ultrasound devices have increased the rate of detection. They are classified as calcification in a mass, calcification outside a mass, or intraductal calcification.
(3) Special cases: They include simple cysts, clustered microcysts, complicated cysts, mass in or on the skin, foreign bodies and implants, lymph nodes (intramammary or axillary), vascular abnormalities (arteriovenous malformations and Mondor’s disease), postsurgical fluid collections, and fat necrosis.
As in MG, the features can be described along with other lesions, involving architectural distortions, duct changes, skin changes (thickening and retraction), and edema. Some of the abovementioned findings have a significant positive or negative predictive value in classifying a finding as malignant and thus determining the BI-RADS category. Characteristics of breast masses that imply a negative predictive value in US are round or oval shape (84%), circumscribed margins (90%), and a parallel orientation (78%). On the other hand, a significant positive predictive value is characterized by an irregular shape (62%), spiculated margins (86%), and a non-parallel orientation (69%) (12). A summary of the important features that should be clearly described for a breast mass in an ultrasound report is demonstrated in Table 1. According to a recent study, because there are many features that need to be described in a breast ultrasound report, which may be confusing to the user, the report can be arranged in a practical, user-friendly manner (13).
| Features | Descriptions |
|---|---|
| Location | Clock face, distanced from the nipple |
| Size a | Measured and expressed for at least two dimensions |
| Shape | Oval, round, and irregular |
| Margin | Circumscribed, indistinct, angular, microlobulated, and spiculated |
| Orientation b | Parallel and non-parallel |
| Echo pattern | Anechoic, hypoechoic, isoechoic, hyperechoic, and heteroechoic |
| Posterior features | None, enhancement, shadowing, and combined pattern |
| Vascularity | Absent, internal, and vessels in rim |
| Elasticity | Soft, intermediate, and hard |
| Bilateral multiple circumscribed masses | Assessed as benign unless one mass is different from others. If description is necessary, every finding should be presented in a separate paragraph, and the breast, location, and size need to be documented. |
a A measure for only important findings, not necessary for small simple cysts; in numerous cysts, only the size and location of the largest cyst are reported.
b It is also defined as horizontal or vertical.
6.3. MRI
Apart from the background parenchymal enhancement, which is classified under the overall breast composition, enhancement can be defined for an individual lesion. In other words, the rate or speed at which the contrast agent enters, remains in, and exits a lesion should be assessed and described. The gadolinium uptake enhancement and washout (kinetic curve assessment - signal intensity (SI)/time curve) is the most important feature in interpretation of a lesion detected on MRI (6). Generally, the following findings should be reported on MRI:
(1) Focus: A unique punctate enhancing dot, usually smaller than 5 mm, with no morphological features on a pre-contrast scan. This is non-specific and may be benign or malignant, depending on the associated features.
(2) Masses: The mass shape and margin are used to differentiate malignant and benign findings (shape: Oval, round, and irregular; margin: Circumscribed and non-circumscribed [irregular and spiculated]; and internal enhancement characteristics: Homogeneous, heterogeneous, rim enhancement, and dark internal septa).
Among the mentioned MRI characteristics of a mass, irregular shape, non-circumscribed margin, and heterogeneous or rim enhancement represent malignancy, while others represent benignity.
(3) Non-mass enhancement (NME): If the enhancement is neither focus nor mass, it is classified as NME. The NME is assessed from two points of view:
• Distribution: Focal, linear, segmental, regional, multiple regions, and diffuse.
• Internal enhancement pattern: Homogeneous, heterogeneous, clumped, and clustered ring.
The internal enhancement patterns of segmental or linear distribution and heterogeneous, clumped, or clustered ring suggest malignancy.
(4) Kinetic curve assessment: By definition, an abnormal enhancement refers to a higher enhancement compared to the surrounding normal background tissue. The kinetic curve is a dynamic measurement tool that monitors the uptake and washout of the contrast in a tissue and helps differentiate malignant from benign lesions. A tumor tends to enhance more rapidly and stronger than the normal surrounding tissue. A kinetic curve represents two main phases:
• Initial phase: It occurs within the first two minutes after contrast injection, or when the curve starts to change. It can be slow, medium, or fast.
• Delayed phase: It occurs after two minutes and may be persistent (an enhancement increase more than 10% over time), plateau (stable), or washout (an enhancement decrease more than 10% over time).
Based on the abovementioned characteristics, a lesion can have one of the following three types of enhancement curves: Type 1 (progressive enhancement pattern) with a 6% risk of malignancy; type 2 (plateau pattern) with a malignancy risk of 7% to 29%; and type 3 (washout pattern) with a malignancy risk of 30% to 77%. Practically, when the two latter types are seen, a lesion should be considered suspicious for malignancy if there are other descriptors, such as shape, margin, and internal enhancement pattern in the context of a mass or if there is suspicious distribution and internal enhancement in the context of NME (14, 15).
The location of a lesion in breast MRI should be described by quadrant and clock-face positions. However, the patient’s prone position during MRI, the patient’s Standing position plus breast compression for MG, and the patient’s supine position for ultrasound and clinical examinations make it challenging to determine the correlation between the findings and clarify the tumor location. Although distance from the nipple can be measured and reported, this measure is not always consistent in different imaging and clinical modalities (6). When additional techniques have been proposed for further assessments, they should be included in the report.
7. Comparison with Previous Examinations
Comparison of the report with the previous examination is important to specify if the finding of concern is stable or has changed over time. Any new finding(s), or increase in size, or changes in the features of existing lesions should be mentioned in the report, as they raise the suspicion of malignancy. In contrast, a decrease in size favors benignity. Practically, comparison is not needed for lesions that have been proven to be benign or are characteristically benign. When a comparison is made, it should be always mentioned in the report; otherwise, the reader assumes that no comparison has been made (4-6). Table 2 is a quick reminder of actions that must be taken when comparing breast US findings with other relevant investigations.
| Considerations | Actions |
|---|---|
| Attention must be paid not to misidentify different lesions as a single one in different imaging modalities. | Match the lesion location a |
| Compare the lesion size b | |
| Correlate the features of the surrounding tissue. | |
| A present US finding corresponds to a lesion found in the physical examination, MG, or MRI. | Explain the correlation in the report. |
| A new lesion is detected in the US image, which has no indication in the physical exam, MG, or MRI. | Describe its novelty in the report. |
| A present US finding corresponds to a lesion found in a previous US. | All detected changes should be documented in the report. |
Abbreviations; MG, mammography; MRI, magnetic resonance imaging; US, ultrasound.
a Adjust for positional changes of MG, MRI, and US.
b If the largest diameter of a probably benign lesion has an increase of more than 20% during six months, biopsy is needed. The patient’s position and the US technique affect the size measurements; a 1 - 2 mm increase in size does not warrant further actions.
8. Composite Reports
They are used when several breast imaging modalities are applied on the same day, namely, MG and ultrasound; it is recommended to report the modalities together with the overall assessment and recommendations. Obviously, some parts of the report should be documented separately for each modality, such as technical considerations, findings, description of breast composition for each modality, and some comparisons. When the results of individual assessments are different, the poorer result should be reported, except when the features of a lesion are typically benign in one modality, while the malignant appearance is non-characteristic based on another imaging modality; in this context, a typical benign diagnosis can be approved (5). MRI is normally reported separately, and its findings are usually correlated with other findings at the time of clinical decision-making.
9. Assessment
Although assessments have been described separately in relevant studies on MG, US, and MRI, they have a very similar overall concept. The final assessment is based on the overall interpretation of imaging findings and assignment of the BI-RADS category. These categories are similar for the three imaging modalities, centered on numbers or scores with definite descriptions. They are described below in the “BI-RADS classification” section.
10. Management
Management refers to recommendations for further investigation or treatment. For example, if a BI-RADS category 5 is assigned, the report should indicate that biopsy is warranted in the absence of clinical contraindications.
11. BI-RADS Classification
The overall BIRADS classification of findings in a breast image and the management strategy that is suggested in BIRADS follows a uniform standard for MG, US and MRI. In general, the BI-RADS system includes seven categories or scores from 0 to 6 (Table 3 and Table 4); category 4 includes three subcategories of a, b, and c (3, 16) for MG and ultrasound.
| Mammography | Ultrasound | MRI | |
|---|---|---|---|
| Indications | BC screening; Further evaluation of a finding; F/U after BC or for benign lesions | Mass evaluation; Complementary dense MG, especially in HR; Targeted for MG or MRI; First imaging when MG is contraindicated; Axilla evaluation, especially in B4, 5, and 6 | BC screening in HR; Further evaluation of BC, especially lobular BC; F/U of the previous lesion; Evaluation of BC after NAC |
| Techniques | Not defined | Scope: Targeted/whole breast; Technique: Transducer frequency; Patient position; Options (eg, Doppler) | A dedicated breast coil; Pulse sequence (T1/T2, fat saturation); Orientation (axial, coronal, and sagittal); Contrast (dynamic scan and intervals); Postprocessing (MIP and subtraction) |
| Breast composition | |||
| CAT a | Almost entirely fatty | Homogenous BE and fat | FGT fat; minimal BPE |
| CAT b | Scattered FGT | Homogenous BE and FG | Scattered FGT; mild BPE |
| CAT c | Heterogeneously dense | Heterogenous BE | Heterogenous FGT; moderate BPE |
| CAT d | Extremely dense | - | Extreme FGT; marked BPE |
| Important findings | Mass: Shape; Margin; Density | Mass: Shape; Orientation; Margin; Echo pattern; Posterior features; Vascularity; Elasticity | Mass: Shape; Margin; Internal enhancement |
| Cal.: Benign; and Suspicious | Cal.: Inside mass; Outside mass; Intraductal | NME: Distribution; Enhancement patterns | |
| Architectural distortion | Others | Others | |
| Asymmetry, focal asymmetry; Global asymmetry; Developing asymmetry | |||
| Others | |||
| Comparison | - New finding | - New finding | - New finding |
| - Stability | - Stability | - Stability | |
| - Change | - Change | - Change | |
| Composite reports | Not defined | Built on all images acquired on the same day; The poorer finding is reported. | Not defined |
| Assessment | B0: Incomplete | B0: Incomplete | B0: Incomplete |
| B1: Negative | B1: Negative | B1: Negative | |
| B2: Benign | B2: Benign | B2: Benign | |
| B3: Probably benign | B3: Probably benign | B3: Probably benign | |
| B4a: Low suspicion | B4a: Low suspicion | B4: Suspicion | |
| B4b: Moderate suspicion | B4b: Moderate suspicion | ||
| B4c: High suspicion | B4c: High suspicion | ||
| B5: Highly suggestive of Mx | B5: Highly suggestive of Mx | B5: Highly suggestive of Mx | |
| B6: Biopsy proven Mx | B6: Biopsy proven Mx | B6: Biopsy proven Mx | |
| Management | B0: Need for the previous/more examinations | B0: Need for the previous/more examinations | B0: Need for the previous/more examinations |
| B1: Routine screening | B1: Routine screening | B1: Routine screening | |
| B2: Routine screening | B2: Routine screening | B2: Routine screening | |
| B3: Short-term F/U, 2 y | B3: Short-term F/U, 2 y | B3: Short-term F/U, 2 y | |
| B4a: Biopsy | B4a: Biopsy | B4: Biopsy | |
| B4b: Biopsy | B4b: Biopsy | ||
| B4c: Biopsy | B4c: Biopsy | ||
| B5: Biopsy | B5: Biopsy | B5: Biopsy | |
| B6: BC treatment | B6: BC treatment | B6: BC treatment |
Abbreviations: ACR, American College of Radiology; B, breast imaging-reporting and data system (BI-RADS); BC, breast cancer; BE, background echotexture; BPE, background parenchymal enhancement; CAT, category; Cal., calcification; esp., especially; FG, fibroglandular; FGT, fibroglandular tissue; F/U, follow-up; HR, high-risk group; MG, mammography; m, month(s); Mx, malignancy; NAC, neoadjuvant chemotherapy; NME, non-mass enhancement; y, year(s).
| Mammography | Ultrasound | MRI | |
|---|---|---|---|
| 1. Indications | Why is the examination performed? | ||
| 2. Techniques | - | Targeted/whole breast imaging (with options including color Doppler) and the position of the patient during US | Pulse sequences; Image orientation; Type, dose, and timing of contrast administration; Postprocessing: MIP and subtraction |
| 3. Breast composition | Categories a-d (from almost entirely fatty to extremely dense) | Categories a-c (from homogeneous fat to heterogeneous echotexture) | Categories a-d (fromminimal to extreme FGT and BPE) |
| 4. Important findings | Mass | ||
| Calcification | Non-mass enhancement | ||
| Architectural distortion | Others | ||
| Asymmetry | |||
| Others | |||
| 5. Comparison with previous/other images | Stability/recent changes of previous findings/a new finding | ||
| 6. Composite reports | Built on all images in one day | ||
| 7. Assessment | B0: Incomplete | ||
| B1: Negative | |||
| B2: Benign | |||
| B3: Probably benign | |||
| B4a: Low suspicion for Mx | |||
| B4b: Moderate suspicion for Mx | |||
| B4c: High suspicion for Mx | |||
| B5: Highly suggestive of Mx | |||
| B6: Biopsy proven Mx | |||
| Management | B0: Recall for additional imaging and/or comparison with previous examinations | ||
| B1: Routine screening | |||
| B2: Routine screening | |||
| B3: Short-term F/U (2 y) | |||
| B4a, B4b, B4c, B5: Biopsy | |||
| B6: Treatment of breast cancer | |||
Abbreviations: ACR, American College of Radiology; B, breast imaging-reporting and data system (BI-RADS); BPE, background parenchymal enhancement; FGT, fibroglandular tissue; F/U, follow-up; Mx, malignancy.
• Category 0: Incomplete; needs additional imaging evaluation; and involves examinations that are non-diagnostic due to a vague finding or sometimes technically incorrect. They should be typically repeated, or further investigation must be performed with another imaging modality.
• Category 1: Negative; involves examinations that are completely normal; and management involves routine screening based on medical history and age.
• Category 2: Benign; includes imaging findings that are absolutely benign; and routine screening is recommended depending on the patient’s age, medical history, and family history.
• Category 3: Probably benign; it includes findings that are probably benign, with a malignancy probability less than 2%. In these cases, management involves follow-up of the lesion every six months up to two years (16). A stable course of change or a downward slope in terms of tumor size, density, and shape converts the BI-RADS category 3 (B3) to BI-RADS category 2 (B2), while an increase in size or change in other features toward suspicious diagnoses converts it to BI-RADS category 4 (B4) or BI-RADS category 5 (B5). Attention should be paid to the correct classification of findings in this category rather than higher categories (17-19).
• Category 4: Suspicious; it is a heterogeneous group in terms of malignancy risk, as it initially included lesions with a cancer probability of 2 to 95%. In the fourth edition of BI-RADS, it was divided into three subcategories for a better assessment of cancer risk: B4a with a low probability for cancer (2 to 10%), B4b with a moderate probability (11 to 50%), and B4c with a high probability (51 to 95%). Despite this subdivision, all three subcategories should be approached similarly, and commonly, biopsy of the observed lesion is needed (16). Rarely, B4a can be converted to B3 based on other imaging findings (20, 21). When a B4a lesion is biopsied, and the histological examination favors a benign lesion, it is usually downgraded to B3. In case of stability in the following six months, finally, a category B2 is assigned. This is not a general rule, and management is based on the clinical and paraclinical parameters.
• Category 5: Highly suspicious; it indicates a very high probability of cancer, which is more than 95% and obviously needs to be evaluated via biopsy of the lesion (16).
• Category 6: Biopsy-proven malignancy; BI-RADS category 6 (B6) is assigned to lesions that have been proven to be malignant in a previous biopsy and require further management (Table 3 and Table 4).
12. BI-RADS at a Glance
The BI-RADS has many descriptors, details, and fine points, each of which is important in understanding and communicating breast imaging results; therefore, none of them should be limited or removed. However, to include all significant parameters in a concise manner that can be easily comprehended, they have been included in a single panel, as shown in Table 3. Therefore, to make it as easy as possible, the main items are summarized in Table 4.