1. Background
Malignant melanoma is a well-recognized mucosal or cutaneous malignancy, with a high potential for an extensive and unexpected metastasis to different organs (1). The current staging/restaging methods are based on the lesion thickness, lymph node involvement, and metastatic status. Different imaging modalities, such as computed tomography (CT) scan, magnetic resonance imaging (MRI), and recently positron emission tomography/CT (PET/CT) scan, have been used as significant diagnostic tools, especially for more advanced diseases (stage III/IV) (2).
The survival rate of patients with malignant melanoma depends on the size and depth of the lesion, as well as the presence of regional and/or distant metastasis (3-5). Advanced malignant melanoma refers to stage III/IV of the disease according to the American Joint Committee on Cancer (AJCC) criteria and includes melanoma with nodal and/or distant metastasis (6, 7). Lymph node and distant metastases may be identified by clinical examination, sentinel lymph node biopsy (SLNB), and imaging techniques. Overall, early diagnosis and treatment can significantly improve prognosis and survival (8, 9).
18F-fluorodeoxyglucose (FDG) PET/CT scan is based on the evaluation of cellular metabolism; the higher glucose metabolism of cancer cells compared to normal cells can be very helpful in the detection of tumor lesions (10). FDG PET/CT scan is now considered a standard method for the initial diagnosis, staging, treatment planning, evaluation of treatment response, and tumor restaging after treatment for many cancers (11). Recent evidence suggests that FDG PET/CT parameters, including the maximum standardized uptake value (SUVmax), are associated with the prognosis of different malignancies. Although FDG PET/CT may be considered an ideal method for the detection of melanoma metastasis, there is still controversy regarding its clinical application. Some studies have demonstrated that FDG PET/CT is a sensitive and accurate method for staging of advanced melanoma (10, 12, 13). There are also few studies evaluating the association between pathological features and FDG PET/CT findings in patients with advanced melanoma (14). pathological features and immunohistochemical (IHC) markers have been integrated in melanoma staging, disease prognosis, and selection of new treatments (15, 16). Therefore, it can be interesting to determine the association between pathological features and FDG PET/CT findings.
2. Objectives
This study aimed to investigate the association between FDG PET/CT scan findings and tumor pathological features in patients with malignant melanoma.
3. Patients and Methods
In this cross-sectional study, the baseline information of adult patients (age > 18 years) with a definitive diagnosis of cutaneous or mucosal melanoma (stage III or IV according to the pathological findings and the AJCC criteria), who were admitted to Masih Daneshvari Hospital (Tehran, Iran) for FDG PET/CT scan during 2016 - 2021, was collected by reviewing their medical records. The demographic information of all patients, including age and sex, as well as their medical history, was also retrieved. The inclusion criteria were as follows: (1) age over 18 years; (2) a definitive diagnosis of advanced melanoma stage III or IV according to the AJCC criteria, confirmed by a histological evaluation; (3) availability of pathological and FDG PET/CT scan findings; and (4) a signed informed consent form. On the other hand, patients who met the following criteria were excluded from the study: (1) a concomitant malignancy or a history of malignancy in the last 10 years; (2) a history of systemic treatment for melanoma or metastasectomy; and (3) unwillingness to continue the study at any stage. The variables under study included age, sex, cutaneous versus mucosal lesions, primary tumor thickness, ulceration, tumor margin, metastasis, Ki-67, and SUVmax.
All PET/CT images were acquired using a Discovery 690 VCT system (GE Healthcare, Milwaukee, USA), equipped with a 64-slice CT scanner (LightSpeed VCT, GE Healthcare, Milwaukee, USA). The calculation of SUVmax was automatically performed on a 4.5 advantage PET/CT workstation in the defined region of interest (ROI) for all lesions. Finally, the association between each pathological feature and FDG PET/CT findings was investigated. This study was approved by our institutional review board (IRB) with the ethical code, IR.SBMU.MSP.REC.1399.564.
3.1. Statistical Analysis
Data were analyzed using SPSS version 22 (released in 2013, IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY, USA). Frequency was calculated for qualitative variables, such as cutaneous versus mucosal lesions, ulceration, tumor margin, and metastasis, and for quantitative variables with a normal distribution, such as the primary tumor thickness, Ki-67, and SUVmax, the mean and standard deviation (SD) were calculated; the median value was compared if the distribution was not normal. Besides, the association of SUVmax and the frequency of metastases with the lesion size and diameter was examined using Pearson’s correlation test. The mean values of the two groups were compared using Student’s t-test and Mann-Whitney test. For multiple-group comparisons, one-way analysis of variance (ANOVA) or Kruskal-Wallis test (or median test) was used for parametric or non-parametric data, respectively. A P-value less than 0.05 was considered statistically significant.
4. Results
A total of 103 patients with malignant melanoma were included in this study, with a mean age of 54.7 ± 16.99 years. Sixty-four patients (62.1%) were male, and 39 (37.9%) patients were female. The baseline demographic and clinical characteristics of the patients are presented in Table 1.
| Factors | No. (%) |
|---|---|
| Sex | |
| Male | 64 (62.1) |
| Female | 39 (37.9) |
| Reasons for PET scan | |
| Initial staging | 24 (23.3) |
| Metastatic evaluation | 46 (44.7) |
| Recurrence | 2 (1.9) |
| Treatment response | 11 (10.7) |
| Restaging | 20 (19.4) |
| Primary tumor location | |
| Sinus | 5 (5.0) |
| Limb | 67 (67.0) |
| Anal | 4 (4.0) |
| Eye | 4 (4.0) |
| Scalp | 11 (11.0) |
| Mouth | 2 (2.0) |
| Chest | 2 (2.0) |
| Esophagus | 1 (1.0) |
| Abdomen | 3 (3.0) |
| Vagina | 1 (1.0) |
| Primary location | |
| Cutaneous | 83 (83.0) |
| Mucosal | 17 (17.0) |
| Brain metastasis | |
| Yes | 2 (2.0) |
| No | 100 (98.0) |
| Bone metastasis | |
| Yes | 13 (12.7) |
| No | 89 (87.3) |
| Lung metastasis | |
| Yes | 49 (48.0) |
| No | 53 52.0 () |
| Liver metastasis | |
| Yes | 11 (10.8) |
| No | 91 (89.2) |
| Regional lymph node metastasis | |
| Yes | 66 (65.3) |
| No | 35 (34.7) |
| Other metastases | |
| Yes | 48 (48.0) |
| No | 52 (52.0) |
| Metastasis type | |
| No metastasis | 18 (17.6) |
| Single metastasis | 24 (23.5) |
| Multiple metastases | 60 (58.8) |
| Type of pathology | |
| Nodular | 16 (34.0) |
| Acral lentiginous melanoma | 25 (53.2) |
| Superficial | 1 (2.1) |
| Spindle | 2 (4.3) |
| Epithelioid | 2 (4.3) |
Abbreviation: PET, positron emission tomography.
The most common pathological types were acral lentiginous melanoma and nodular melanoma with frequencies of 53.2% and 34%, respectively. The mean thickness of the primary tumor was 5.03 mm, and the mean mitotic rate was 3.32 per mm3. In most cases (44.7%), the reason for FDG PET/CT request was metastasis evaluation. Lymph node metastasis was the most common type of metastasis, as reported in 65.3% of cases. The prevalence of bone, liver, and brain metastases was also estimated at 12.7%, 10.8%, and 2%, respectively. More than half of the patients (58.8%) had multiple metastases. The metastasis data were presented based on the current PET/CT findings, and no previous data were included. The mean SUVmax values for metastases and pathological features are shown in Table 2. There was a significant association between the SUVmax of lung metastasis and the primary tumor thickness (P = 0.029). Associations between the pathological features and the SUVmax of primary tumor and metastasis, based on Spearman’s test, are described in Table 3.
| Parameters | Valid N | Mean ± SD | Median | 25th percentile | 75th percentile |
|---|---|---|---|---|---|
| Age | 102 | 54.70 ± 16.99 | 56.50 | 42.00 | 68.00 |
| SUVmax of involvement in the primary lesion | 63 | 7.89 ± 5.83 | 6.10 | 3.20 | 10.50 |
| SUVmax of brain metastasis | 0 | - | - | - | - |
| SUVmax of bone metastasis | 12 | 5.50 ± 5.71 | 4.35 | 0.00 | 9.00 |
| SUVmax of lung metastasis | 87 | 2.80 ± 4.27 | 0.00 | 0.00 | 4.20 |
| SUVmax of liver metastasis | 83 | 0.76 ± 2.85 | 0.00 | 0.00 | 0.00 |
| SUVmax of regional lymph node metastasis | 100 | 3.80 ± 4.94 | 2.75 | 0.00 | 5.85 |
| Primary tumor thickness (mm) | 55 | 5.03 ± 7.61 | 4.00 | 1.60 | 5.00 |
| Mitotic rate | 25 | 3.32 ± 4.33 | 1.00 | 1.00 | 4.00 |
| IHC marker: Ki-67 | 22 | 20 ± 20 | 20 | 1 | 30 |
Abbreviations: SUV, standardized uptake value; IHC, immunohistochemical assay; Ki-67, mitotic index indicating the number of cells dividing.
| Variables | SUVmax of involvement in the primary PET | SUVmax of bone metastasis | SUVmax of lung metastasis | SUVmax of liver metastasis | SUVmax of regional lymph node metastasis |
|---|---|---|---|---|---|
| Spearman’s correlation | |||||
| N | 62 | 12 | 85 | 81 | 100 |
| Primary tumor thickness (mm) | |||||
| Correlation coefficient | 0.232 | 0.289 | 0.316 | 0.153 | 0.117 |
| P-value | 0.216 | 0.637 | 0.029 a | 0.317 | 0.397 |
| N | 30 | 5 | 48 | 45 | 55 |
| Mitotic rate | |||||
| Correlation coefficient | -0.250 | -0.014 | -0.271 | 0.015 | |
| P-value | 0.410 | 0.956 | 0.276 | 0.943 | |
| N | 13 | 1 | 19 | 18 | 25 |
| IHC marker: Ki-67 | |||||
| Correlation coefficient | 0.039 | -1.000 b | 0.070 | 0.273 | |
| P-value | 0.900 | NA | 0.789 | 0.230 | |
| N | 13 | 3 | 17 | 15 | 21 |
Abbreviations: SUV, standardized uptake value; PET, positron emission tomography; IHC, immunohistochemical assay.
a Statistically significant.
b An inverse association.
The analysis of the association between the SUVmax and free margin revealed a significant association between the SUVmax of lung metastasis and free margin (P = 0.047). In other words, patients without a free margin had a significantly higher mean SUVmax of lung metastasis compared to patients with a free margin (3.12 vs. 1.69; P = 0.047). Regarding the association between the SUVmax and ulceration, a significant relationship was found between the SUVmax of lung metastasis and ulceration (P = 0.041). In other words, patients with ulceration had a significantly higher mean SUVmax of lung metastasis compared to patients without ulceration (3.28 vs. 1.81; P = 0.041). However, no significant association was observed between the SUVmax of each metastasis and regional lymph node involvement.
Moreover, the associations between the primary tumor location and pathological features are shown in Table 4. There was a significant association between the primary tumor location and the primary tumor thickness (P = 0.021). In other words, the mean primary tumor thickness in patients with mucosal involvement was significantly higher than that of patients with skin involvement (15.60 vs. 3.97; P = 0.021). In contrast, the mean Ki-67 index was significantly higher in patients with skin involvement compared to patients with mucosal involvement (22.0 vs. 1.0; P = 0.008). Nevertheless, no significant association was observed between the primary tumor location (cutaneous vs. mucosal) and the tumor mitotic rate.
Abbreviation: IHC, immunohistochemical assay.
a Statistically significant.
Considering the association between the metastasis type (no metastasis vs. single or multiple metastases) and pathological features, no significant association was found between the metastasis type and free margin, ulceration, or Ki-67 index. Associations between the metastasis type and pathological features are demonstrated in Table 5.
| Variables | Metastasis type | P-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No metastasis | Single metastasis | Multiple metastases | ||||||||
| Valid N | Mean ± SD | Median | Valid N | Mean ± SD | Median | Valid N | Mean ± SD | Median | ||
| Primary tumor thickness (mm) | 9 | 3.26 ± 1.97 | 4.00 | 11 | 2.74 ± 1.51 | 3.00 | 35 | 6.21 ± 9.30 | 4.00 | 0.322 |
| Mitotic rate | 5 | 2.80 ± 2.17 | 2.00 | 5 | 3.20 ± 3.90 | 1.00 | 15 | 3.53 ± 5.13 | 1.00 | 0.887 |
| IHC marker: Ki-67 | 6 | 14 ± 14 | 10 | 5 | 22 ± 21 | 30 | 10 | 25 ± 24 | 20 | 0.659 |
Abbreviation: IHC, immunohistochemical assay.
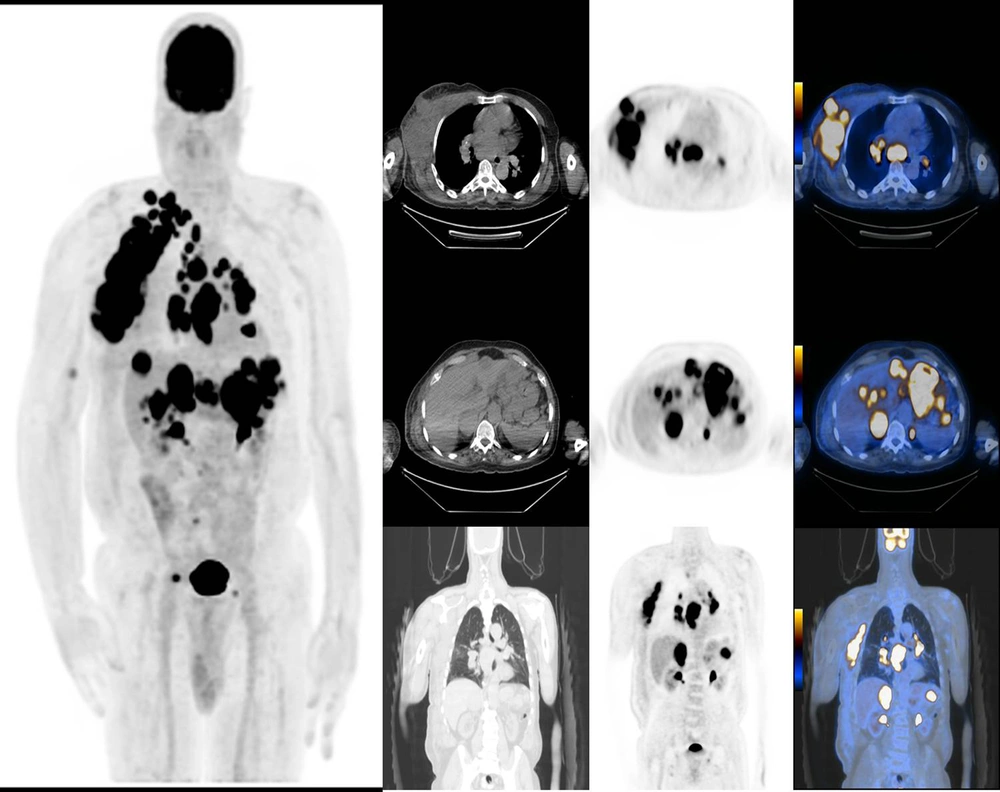
Moreover, the relationship between the primary tumor location and the SUV values of primary tumor and metastases is shown in Table 6. The mean SUVmax of lung metastasis was significantly higher in mucosal melanoma compared to cutaneous melanoma. However, the mean SUVmax values of other metastases (bone, liver, and lymph node), and even that of the primary lesion itself, were not significantly different between the two groups (Figures 1 & 2).
| Variables | Primary location | P-value | |||||
|---|---|---|---|---|---|---|---|
| Cutaneous | Mucosal | ||||||
| Valid N | Mean ± SD | Median | Valid N | Mean ± SD | Median | ||
| SUVmax of involvement in the primary PET | 52 | 7.40 ± 5.49 | 5.85 | 8 | 11.10 ± 7.68 | 8.10 | 0.123 |
| SUVmax of lung metastasis | 10 | 4.03 ± 4.89 | 2.50 | 2 | 12.85 ± 3.75 | 12.85 | 0.049 a |
| SUVmax of bone metastasis | 70 | 2.61 ± 4.16 | 0.00 | 15 | 3.38 ± 4.64 | 0.00 | 0.476 |
| SUVmax of liver metastasis | 68 | 0.63 ± 2.35 | 0.00 | 14 | 1.48 ± 4.69 | 0.00 | 0.401 |
| SUVmax of regional lymph node metastasis | 82 | 3.33 ± 4.26 | 2.50 | 15 | 6.80 ± 7.46 | 6.00 | 0.135 |
Abbreviations: SUV, standardized uptake value; PET, positron emission tomography.
a Statistically significant.
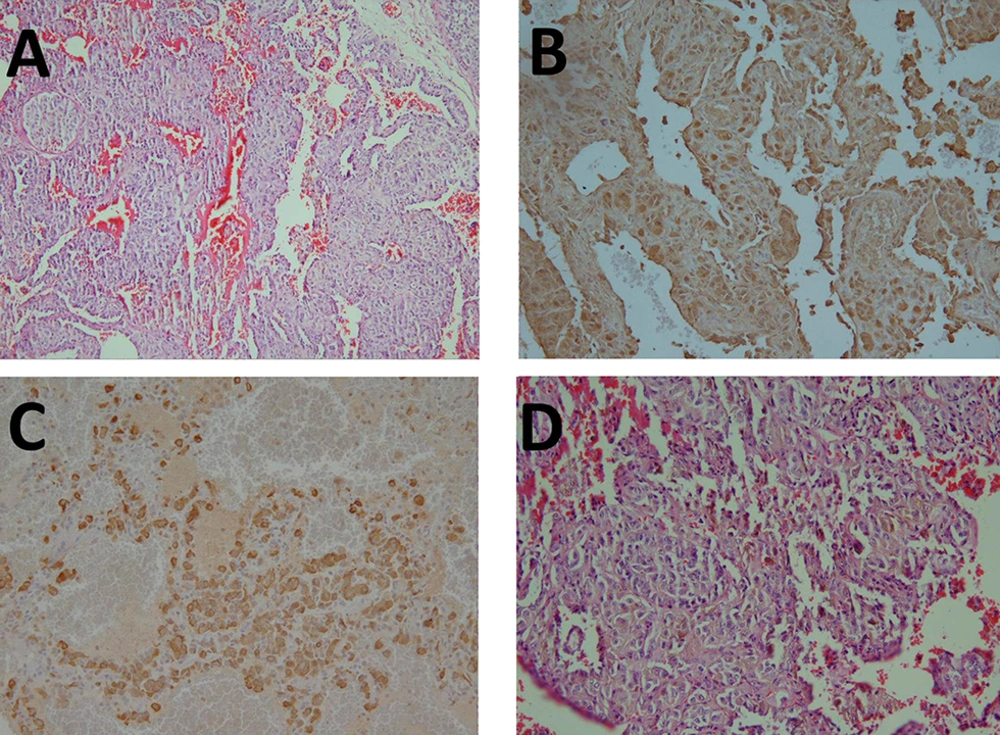
A, A lung tissue with interstitial infiltration of atypical cells with a clear cytoplasm; B, Some tumor cells are positive for S-100 protein; C, All cells are positive for human melanoma black-45 (HMB45) as a monoclonal antibody that reacts against an antigen in melanocytic tumors, such as melanomas; D, Some pigment deposition is present.
5. Discussion
This study aimed to investigate the association between FDG PET/CT findings and the pathological features of primary tumors in melanoma patients. The results revealed that patients with a free margin had a significantly lower SUVmax of lung metastasis compared to patients without a free margin. Patients with ulceration also had a significantly higher SUVmax of lung metastasis compared to those without ulceration. There was also a significant relationship between the SUVmax of lung metastasis and the primary tumor thickness. In other words, an increase in the primary tumor thickness was associated with a higher SUVmax of lung metastasis.
Moreover, the present findings demonstrated that patients with single metastasis and multiple metastases had higher SUVmax values than those without metastasis. A significant association was also observed between the location of melanoma lesion (cutaneous versus mucosal), the level of Ki-67 protein (an IHC marker), and the primary tumor thickness. These results indicate that increased tumor thickness, ulceration, and margin involvement on pathology reports are likely to be associated with higher SUVmax values, especially in lung metastases. Therefore, it can be interpreted that higher SUVmax values are potentially associated with more significant high-risk pathological features and a poorer prognosis.
A high SUV in lymph node metastasis is an independent negative prognostic factor for disease-free survival; however, it has no impact on the overall survival (17). In a previous study, Rasmussen et al. examined the association between the expression of IHC markers, including Bcl-2, β-tubulin-1 and 2, EGFR, Ki-67, and glutathione-s-transferase, and PET parameters in head and neck squamous cell carcinoma. They found a significant negative relationship between the SUVmax and the expression of Bcl2 and β-tubulin I and II. They concluded that there was a significant relationship between the expression of IHC parameters in primary tumors and FDG PET/CT results (18).
In another study, Bitencourt et al. evaluated the relationship between the expression of IHC biomarker and PET results in 50 patients with breast cancer. Their findings showed a significant positive relationship between the SUVmax and histology type, histology grade, molecular subtype, tumor diameter, mitotic index, and Ki-67 expression (19). It is generally accepted that some pathological features of melanoma patients are associated with positive FDG PET/CT findings. These pathological features include a mitotic rate > 3/mm2, tumor thickness > 4 mm, regional lymphadenopathy, and bleeding/ulceration (5, 20). PET is more useful in detecting distant metastasis than regional metastasis, given the established role of SLNB (21).
FDG PET/CT may be a highly useful tool for the surveillance of melanoma patients. For the follow-up of patients with advanced stage melanoma (stage III/IV), the National Comprehensive Cancer Network (NCCN) guidelines recommend imaging (including PET/CT) every three to 12 months to screen for recurrence or metastatic disease. However, routine imaging to screen asymptomatic cases is not recommended after three to five years (22). According to our literature review, few researchers have investigated the association between FDG PET/CT scan findings and pathological features of patients with malignant melanoma. This may be considered the novelty of our study, although we could only demonstrate few associations, and further studies are strongly recommended.
In conclusion, based on on the results of the present study, there may be an association between FDG PET/CT findings and some pathological features of melanoma patients. Factors, such as the primary tumor thickness, cutaneous versus mucosal tumor location, metastasis type, free margin, and ulceration, were significantly associated with PET/CT findings. Further multicenter and community-based studies are recommended with a larger sample size to obtain more reliable results.
.jpg)