1. Background
Endometriosis, as a multiorgan and often painful disorder, is recognized as one of the most common gynecological diseases, associated with various morbidities. It is defined as the abnormal growth of endometrium-type tissue outside the endometrial cavity (1). The most common sites for endometriosis include the ovaries, peritoneum, and uterosacral ligament, while it less frequently involves the bladder, vagina, and gastrointestinal tract (1, 2). Deeply infiltrating endometriosis (DIE) is a specific type of endometriosis, penetrating more than 5 mm below the peritoneal surface. Laparoscopy and subsequent histological confirmation are the mainstay for a definitive diagnosis. However, there are many challenges in the application of imaging modalities. Among available imaging modalities, magnetic resonance imaging (MRI) has been postulated as the most accurate method for DIE mapping (3).
So far, various imaging modalities have been recommended in the literature to diagnose and localize DIE, including transvaginal sonography (TVS), transrectal sonography (TRS) or rectal endoscopic sonography (RES), and MRI (4, 5). Deeply infiltrating endometriosis in the uterosacral ligament, visceral wall, or pouch of Douglas (POD) (responsible for POD obliteration) is associated with serosal adhesion, fibrosis, and rectal DIE and can be identified via TVS (6, 7). Moreover, POD obliteration is associated with DIE in the posterior pelvic compartment (PPC).
Transvaginal sonography is recommended as the first-line imaging modality to diagnose ovarian and bladder endometriosis (1). On the other hand, ultrasound is well known for its high value in the diagnosis of ovarian endometrioma (OE), and observation of OE in TVS may raise the suspicion of endometriosis (8, 9). Additionally, identification of ovarian fixation on TVS through visualization of a not freely mobile ovary (under gentle probe pressure) is strongly associated with the detection of endometriosis via laparoscopy (10). These findings all emphasize that preoperative TVS, as a practical high-yield diagnostic tool for patients with suspicion of DIE/POD obliteration, may waive the need for laparoscopy or exploratory laparotomy in unnecessary cases.
2. Objectives
This study aimed to determine the accuracy of TVS in the diagnosis of DIE and POD obliteration with regard to the presence or absence of OE.
3. Patients and Methods
The current study was approved by the local institutional ethics committee. In this analytical study on prospectively collected data, 110 clinically suspected cases of DIE or POD obliteration, who were referred to our tertiary care teaching health center during 2020 - 2021 and were scheduled for a laparoscopic evaluation, were included. All patients underwent TVS before laparoscopy. After systematically examining the uterus, ovaries, fallopian tube, and other relevant pelvic structures via TVS and laparoscopy, the accuracy of these two modalities in diagnosis of DIE and POD obliteration was assessed and compared, with pathological confirmation as the gold standard. Our findings were further stratified based on the presence, size, laterality, and location of OE, as well as the presence of hydro- or hematosalpinx, peritoneal cysts, and pelvic adhesions.
3.1. Study Population
This cross-sectional study was conducted in a tertiary teaching center with highly skilled staff for the management of endometriosis. Consecutive patients with clinically suspected pelvic endometriosis, who were scheduled for laparoscopy, were asked to join the study after explaining the study plan and obtaining written informed consent. Information leaflets explaining the study objectives and methods were given to all eligible participants. Patients unable to undergo a TVS exam (e.g., in case of virginity or TVS avoidance due to procedural anxiety) were excluded from the study.
3.2. Procedures
Attending clinicians took the clinical history of all patients. Transvaginal sonography exams were performed by two radiologists who were highly experienced in gynecological ultrasonography (with seven and 25 years of gynecological ultrasonography experience, respectively) and blinded to the patients’ history. Discrepancies in the results were resolved by consensus. All laparoscopic surgeries were performed by a laparoscopic surgeon (with 20 years of experience), who was highly skilled in reproductive surgeries and management of advanced endometriosis.
3.2.1. Transvaginal Sonography
All participants underwent TVS examination in the dorsal lithotomy position, using a high-frequency/high-resolution transvaginal volume probe with a systematic approach, involving: (1) visualization of the uterus in the coronal and sagittal planes; (2) searching the ovaries for OE and assessing the ovarian fixation; (3) visualization of fallopian tubes for hydrosalpinx, hematosalpinx, and adhesion; and (4) inspection of the rest of the pelvis for peritoneal cysts and signs of adhesion. Ovarian endometrioma was reported for any thick-walled ovarian cyst with a homogeneous low-level internal echo and measured by dividing the sum of diameters (inner to inner) in three orthogonal planes by three (11).
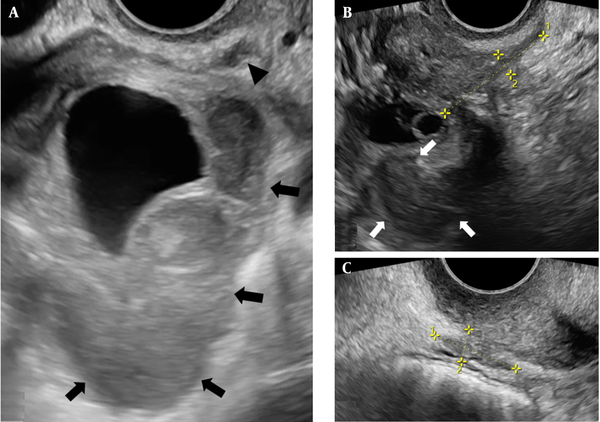
To evaluate pelvic adhesions, the ovarian and uterine mobility was assessed using a bimanual examination (by applying gentle pressure on the ovary or cervix with a vaginal probe and alternating abdominal pressure on the ovary or uterine fundus by the sonographer’s free hand), followed by visualization of free or limited motility of the ovary and uterus across adjacent structures. Hypoechoic or isoechoic solid nodules/masses with irregular margins that were tender on palpation and fixed to the surrounding pelvic structures were labeled as DIE (Figure 1) (12, 13). Also, focal or diffuse hypoechoic thickening of the rectosigmoid colon muscularis propria (thickness > 3 mm), sometimes protruding into the bowels, was considered as rectosigmoid DIE (14). The presence of lesions was recorded in a database file location-wise, using a spreadsheet in Microsoft Excel for Windows.
A, Transvaginal sonography (TVS) presents a sizable right-sided ovarian endometrioma (OE) with a thick echogenic wall and low-level internal echogenicity (arrows) along with an adjacent ipsilateral uterosacral ligament deeply infiltrating endometriosis (DIE) plaque (arrowhead). The DIE plaque has an angulated and irregular margin. B and C, TVS demonstrates an endometrioma (arrows) with adjacent irregular, amorphous, elongated, and hypoechoic foci of DIE deposition between clipper
3.2.2. Laparoscopy
Pre-procedural and procedural planning was performed for any individual case according to in-depth insights into the pelvic anatomy by TVS examinations. Patients were asked not to eat or drink for eight hours before laparoscopy. The procedure was performed under general anesthesia in the steep Trendelenburg position. The abdominopelvic cavity was inflated with carbon dioxide using a cannula (placed through a subumbilical incision), increasing the intraperitoneal pressure up to 13 - 14 mmHg. Laparoscopy was performed according to a systematic approach not to miss small endometriotic deposits. Approaching from the left side, the left ureter was dissected and inspected for any signs of endometriosis. The central pelvis was evaluated for the presence of rectosigmoid endometriosis, pelvic adhesions, or peritoneal cysts.
Finally, the right ureter was dissected to search for endometriotic lesions. Endometriotic deposits or nodules, if found, were removed in any stage through a sharp incision using laparoscopic scissors. If present, pelvic adhesions were excised, and peritoneal/ovarian cystectomy or myomectomy was followed by intraabdominal suturing. In case of a posterior pelvic adhesion, both ovaries were suspended from the ipsilateral round ligaments to decrease the risk of subsequent adhesiogenesis.
3.3. Statistical Analysis
Statistical analysis was performed using SPSS version 22.0 (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp). The accuracy of TVS for each site of involvement was examined by measuring sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR+) and negative likelihood ratio (LR−). Moreover, Fisher’s exact test was used to evaluate the relationship between TVS and laparoscopic findings of DIE, both in the anterior pelvic compartment (APC) and PPC. The level of agreement was calculated based on kappa coefficients (κ), with κ values of 0.81 - 1.0, 0.61 - 0.80, 0.41 - 0.60, 0.21 - 0.40, and < 0.20 representing very good, good, moderate, fair, and poor agreement, respectively. In all tests, a P-value less than 0.05 was considered statistically significant.
4. Results
This study was conducted on 110 patients, with a mean age of 37.20 ± 7.16 years (mean ± SD), ranging from 18 to 52 years. Right-sided OE was detected in 73 (66.4%) patients on both TVS and laparoscopy, with an average size of 48.6 ± 20.9 mm (range, 6 - 120 mm). Left-sided OE was recorded in 74 (67.3%) patients on both TVS and laparoscopy, with an average size of 21.2 ± 48.7 mm (range, 10 - 130 mm). Other descriptive findings are presented in Table 1.
| Variables | Ultrasound (n = 110) | Laparoscopy (n = 110) | P-value |
|---|---|---|---|
| Laterality | |||
| Right ovary | 0.662 b | ||
| Normal | 37 (33.6) | 35 (31.8) | |
| OE | 73 (66.4) | 75 (68.2) | |
| Left ovary | |||
| Normal | 36 (32.7) | 32 (29.1) | |
| OE | 74 (67.3) | 78 (70.9) | |
| Number | |||
| Number of OEs in the right ovary | 0.127 | ||
| 1 | 51 (69.9) | 43 (57.3) | |
| > 1 | 22 (30.1) | 32 (42.7) | |
| Number of OEs in the left ovary | 0.283 | ||
| 1 | 56 (75.7) | 52 (66.7) | |
| > 1 | 18 (24.3) | 26 (33.3) | |
| Compartment involved | |||
| APC | 0.153 | ||
| Negative | 96 (85.7) | 88 (78.6) | |
| B. dome | 3 (2.7) | 4 (3.6) | |
| B. base | 12 (10.7) | 17 (15.2) | |
| B. trigone | 1 (0.9) | 3 (2.7) | |
| PPC | 0.315 | ||
| Negative | 36 (22.6) | 26 (16.4) | |
| RVS | 1 (0.6) | 1 (0.6) | |
| DU | 4 (2.5) | 13 (8.2) | |
| USL | 70 (44) | 66 (41.5) | |
| PVF | 1 (0.6) | 1 (0.6) | |
| VW | 1 (0.6) | 0 (0) | |
| LR | 7 (4.4) | 3 (1.9) | |
| UR | 28 (17.6) | 30 (18.9) | |
| RS | 11 (6.9) | 19 (11.9) | |
| Other findings | |||
| Hydrosalpinx | 0.423 | ||
| Negative | 98 (89.1) | 94 (85.5) | |
| Positive | 12 (10.9) | 16 (14.5) | |
| Hematosalpinx | 0.446 | ||
| Negative | 108 (98.2) | 105 (95.5) | |
| Positive | 2 (1.8) | 5 (4.5) | |
| Evidence of pelvic adhesion | 0.027 | ||
| Negative | 34 (30.9) | 19 (17.3) | |
| Positive | 76 (69.1) | 91 (82.7) | |
| Peritoneal cyst | < 0.0001 | ||
| Negative | 80 (72.7) | 107 (97.3) | |
| Positive | 30 (27.3) | 3 (2.7) |
Comparison of the Presence, Number, and Location of Ovarian Endometrioma and Other Associated Findings on Sonography and Laparoscopy a
According to Table 2, TVS and laparoscopy were significantly correlated with the observation of DIE and POD obliteration in the APC, regardless of the presence, laterality, or size of OE (P < 0.0001 for the presence or absence of OE; P < 0.0001 for uni- or bilateral OE; and P < 0.0001 for OE size ≤ 48 or > 48 mm). A similar finding was reported for DIE and POD obliteration in PPC (P < 0.0001 for OE presence and P = 0.006 for OE absence; P < 0.0001 for uni- or bilateral OE; and P = 0.002 for OE≤48 mm and P < 0.0001 for OE > 48 mm).
| Laparoscopy | P-value a | Kappa coefficient | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative | B. dome | B. base | B. trigone | |||||||
| DIE in APC and POD obliteration with/without/with and without OE | ||||||||||
| TVS | < 0.0001/n b/< 0.0001 | 0.708/n b /0.711 | ||||||||
| Negative | 80/7/87 | 1/0/1 | 6/0/6 | 2/0/2 | ||||||
| B. dome | 0/0/0 | 3/0/3 | 0/0/0 | 0/0/0 | ||||||
| B. base | 1/0/1 | 0/0/0 | 11/0/11 | 0/0/0 | ||||||
| B. trigone | 0/0/0 | 0/0/0 | 0/0/0 | 1/0/1 | ||||||
| DIE in PPC and POD obliteration with/without/with and without OE | ||||||||||
| Negative | RVS | DU | USL | PVF | LR | UR | RS | |||
| TVS | < 0.0001/0.006/< 0.0001 | 0.616/0.859/0.632 | ||||||||
| Negative | 24/2/26 | 0/n/0 | 0/0/0 | 3/0/3 | 0/n/0 | 0/n/0 | 5/0/5 | 2/n/2 | ||
| RVS | 0/n/0 | 0/n/0 | 0/n/0 | 0/n/0 | 0/n/0 | 0/n/0 | 0/n/0 | 1/n/1 | ||
| DU | 0/0/0 | 0/n/0 | 3/1/4 | 0/0/0 | 0/n/0 | 0/n/0 | 0/0/0 | 0/n/0 | ||
| USL | 0/0/0 | 1/n/1 | 6/0/6 | 54/3/57 | 0/n/0 | 0/n/0 | 2/1/3 | 3/n/3 | ||
| PVF | 0/n/0 | 0/n/0 | 0/n/0 | 0/n/0 | 0/n/0 | 0/n/0 | 0/n/0 | 1/n/1 | ||
| VW | 0/n/0 | 0/n/0 | 0/n/0 | 1/n/1 | 0/n/0 | 0/n/0 | 0/n/0 | 0/n/0 | ||
| LR | 0/n/0 | 0/n/0 | 0/n/0 | 0/n/0 | 0/n/0 | 2/n/2 | 4/n/4 | 0/n/0 | ||
| UR | 0/0/0 | 0/n/0 | 2/0/2 | 4/1/5 | 0/n/0 | 0/n/0 | 15/3/8 | 4/n/4 | ||
| RS | 0/n/0 | 0/n/0 | 1/n/1 | 1/n/1 | 1/n/1 | 0/n/0 | 0/n/0 | 8/n/8 | ||
| DIE in APC and POD obliteration in unilateral/bilateral OE | ||||||||||
| Negative | B. dome | B. base | B. trigone | |||||||
| TVS | < 0.0001/< 0.0001 | 0.861/0.592 | ||||||||
| Negative | 46/34 | 1/0 | 0/6 | 0/2 | ||||||
| B. dome | 0/0 | 2/1 | 0/0 | 0/0 | ||||||
| B. base | 1/0 | 0/0 | 4/7 | 0/0 | ||||||
| B. trigone | 0/n | 0/n | 0/n | 1/n | ||||||
| DIE in PPC and POD obliteration in unilateral/bilateral OE | ||||||||||
| Negative | RVS | DU | USL | PVF | LR | UR | RS | |||
| TVS | < 0.0001/< 0.0001 | 0.652/0.577 | ||||||||
| Negative | 17/7 | n/0 | 0 / 0 | 1 /2 | n/0 | 0 / 0 | 2 / 3 | 2 / 0 | ||
| RVS | 0/n | n/n | 0/n | 0/n | n/n | 0/n | 0/n | 1/n | ||
| DU | n/0 | n/0 | n/3 | n/0 | n/0 | n/0 | n/0 | n/0 | ||
| USL | 0/0 | n/1 | 2/4 | 22/32 | n/0 | 0/0 | 1/1 | 2/1 | ||
| VW | 0/n | n/n | 0/n | 1/n | n/n | 0/n | 0/n | 0/n | ||
| PVF | n/0 | n/0 | n/0 | n/0 | n/0 | n/0 | n/0 | n/1 | ||
| LR | 0/0 | n/0 | 0/0 | 0/0 | n/0 | 1/2 | 2/2 | 0/0 | ||
| UR | 0/0 | n/0 | 1/1 | 1/3 | n/0 | 0/0 | 6 / 9 | 0 / 4 | ||
| RS | n/0 | n/0 | n/1 | n/0 | n/1 | n/0 | n/0 | n/4 | ||
| DIE in APC and POD obliteration in ≥ 48 mm/< 48 mm OE | ||||||||||
| Negative | B. dome | B. base | B. trigone | |||||||
| TVS | < 0.0001/< 0.0001 | 0.727/0.683 | ||||||||
| Negative | 43/37 | 1/0 | 2/4 | 2/0 | ||||||
| B. dome | 0/n | 3/n | 0/n | 0/n | ||||||
| B. base | 0/1 | 0/0 | 5/6 | 0/0 | ||||||
| B. trigone | n/0 | n/0 | n/0 | n/1 | ||||||
| DIE in PPC and POD obliteration in ≥ 48 mm/< 48 mm OE | ||||||||||
| Negative | RVS | DU | USL | PVF | LR | UR | RS | |||
| TVS | 0.002/< 0.0001 | 0.577/0.662 | ||||||||
| Negative | 11/13 | n/0 | 0/ 0 | 2/ 1 | n/0 | 0/ 0 | 3/ 2 | 0/ 2 | ||
| RVS | 0/0 | n/0 | 0/3 | 0/0 | n/0 | 0/0 | 0/0 | 1/0 | ||
| DU | n/0 | n/1 | n/3 | n/25 | n/0 | n/0 | n/2 | n/1 | ||
| USL | 0/0 | n/0 | 3/0 | 29/0 | n/0 | 0/1 | 0/2 | 2/0 | ||
| VW | 0/n | n/n | 0/n | 1/n | n/n | 0/n | 0/n | 0/n | ||
| PVF | 0/n | n/n | 0/n | 0/n | n/n | 0/n | 0/n | 1/n | ||
| LR | 0/n | n/n | 0/n | 0/n | n/n | 2/n | 2/n | 0/n | ||
| UR | 0/0 | n/0 | 2/0 | 2/2 | n/0 | 0/0 | 8/7 | 4/0 | ||
| RS | 0/0 | n/0 | 1/0 | 1/0 | n/1 | 0/0 | 0/0 | 5/3 | ||
Agreement Between Transvaginal Sonography and Laparoscopic Findings for the Detection of Deeply Infiltrating Endometriosis, Stratified by the Involved Compartment and Location and the Presence, Laterality, and Size of Ovarian Endometrioma
Transvaginal sonography was the least sensitive modality (53.8%) to identify DIE in APC when there was accompanying OE, measuring ≤ 48 mm in size. On the other hand, it was the most sensitive modality (100%) for identifying DIE in PPC when OE was absent; it was also highly specific for the detection of DIE (97.4 - 100%). The PPV and NPV of TVS for DIE diagnosis were estimated at 87.5 - 100% and 58.3 - 100%, respectively, depending on the pelvic compartment and location involved. Based on the results, TVS was 100% accurate in diagnosing DIE and POD obliteration in PPC when there was no OE, while it was only 82% accurate for demarcating DIE in APC when there were bilateral OEs (Table 3).
| OE status and DIE location | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | Kappa coefficient |
|---|---|---|---|---|---|---|
| Presence | ||||||
| Presence | ||||||
| APC | 58.3 | 98.7 | 93.3 | 88.9 | 89.5 | 0.658 |
| PPC | 92 | 100 | 100 | 70.6 | 93.3 | 0.787 |
| Absence | ||||||
| APC | - | - | - | - | - | - |
| PPC | 100 | 100 | 100 | 100 | 100 | 100 |
| Presence and absence | ||||||
| APC | 58.3 | 98.9 | 93.3 | 89.7 | 90.2 | 0.662 |
| PPC | 92.5 | 100 | 100 | 72.2 | 93.7 | 0.801 |
| Laterality | ||||||
| Unilateral | ||||||
| APC | 87.5 | 97.8 | 87.5 | 97.8 | 96.4 | 0.854 |
| PPC | 90 | 100 | 100 | 77.3 | 92.5 | 0.820 |
| Bilateral | ||||||
| APC | 77.7 | 100 | 100 | 79.1 | 82 | 0.514 |
| PPC | 93.3 | 100 | 100 | 58.3 | 93.9 | 0.705 |
| Size | ||||||
| ≥ 48 mm | ||||||
| APC | 53.8 | 100 | 100 | 87.8 | 89.3 | 0.642 |
| PPC | 92.8 | 100 | 100 | 68.8 | 93.8 | 0.779 |
| < 48 mm | ||||||
| APC | 63.6 | 97.4 | 87.5 | 90.2 | 89.8 | 0.675 |
| PPC | 91.1 | 100 | 100 | 72.2 | 92.8 | 0.794 |
Sensitivity, Specificity, Positive Predictive Value, Negative Predictive Value, and Accuracy of Transvaginal Sonography for the Diagnosis of Deeply Infiltrating Endometriosis and Pouch of Douglas Obliteration Compared to Laparoscopy
The agreement of TVS and laparoscopic findings for the diagnosis of DIE or POD obliteration roughly fell within the moderate agreement range of kappa coefficients. Agreement was very good in the evaluation of DIE and POD obliteration in APC if the patient had unilateral OE; a similar finding was reported in the evaluation of DIE and POD obliteration in PPC if the patient showed no OE on TVS.
The accuracy, sensitivity, and NPV of TVS for the detection of endometriosis in APC were estimated at 89.5%, 58.3%, and 88.9%, respectively, and the corresponding values for PPC were 93.3%, 92%, and 70.6% respectively.
5. Discussion
Deeply infiltrating endometriosis predominantly involves women of reproductive age. Early detection of DIE via imaging facilitates a timely treatment to alleviate the patient’s symptoms and increase their quality of life and chance of conception (15). The prevalence of DIE and associated conditions, which impose a great burden on patients and healthcare systems, has prompted extensive research in this area. Generally, a definite diagnosis requires histological confirmation and laparoscopy. Nonetheless, imaging plays a crucial role in establishing an initial diagnosis in a relevant clinical context and greatly assists in preoperative mapping. In this regard, TVS, as a well-accepted, rapid, cost-effective, widely available, and non-invasive diagnostic method, has been shown to be advantageous (1).
Previous studies have reported a higher frequency of left-sided endometriomas (16, 17), while some research, similar to the present study, did not confirm this finding (18). Single endometrioma (on either side) was 2 - 3 times more common than multiple OEs. TVS was found to detect more single OEs than laparoscopy, while the latter found more multiple OEs than the former. According to the present findings, the most common extraovarian sites for DIE, found on both TVS and laparoscopy, were the uterosacral ligament (USL) in PPC and the bladder base in APC; these findings are comparable to those of previous studies (19).
The current results revealed that the sensitivity, PPV, accuracy, and to a lesser extent, specificity of TVS for the detection of DIE was higher in PPC than APC; on the contrary, NPV was higher for APC lesions. In this regard, Holland et al. reported that the sensitivity of TVS for the diagnosis of endometriosis in PPC was as low as 10 - 50% (18). Conversely, based on the current results, TVS was quite sensitive for detecting DIE in PPC (91.1 - 100%). According to our findings, DIE and POD obliteration were accurately identifiable via TVS, regardless of the presence, size, or laterality of pelvic endometriosis. On the contrary, Leonardi et al. found a higher TVS detection rate for DIE when OE was present (17). They declared that in cases without OE, the detection rate of TVS was lower to an extent which is not negligible (17).
In line with previous investigations (20), in the present study, the presence of OE on TVS could indicate more severe endometriosis; however, TVS could still detect DIE or even POD obliteration with acceptable accuracy in cases without OE. There was an acceptable agreement between TVS and laparoscopic findings for different DIE features and sites of involvement. The current findings are consistent with the results of other studies (21) and underscore the accuracy of TVS to detect pelvic endometriosis.
Diagnosis of USL endometriosis using TVS has always been a major challenge in clinical practice, as it is strongly related to the sonographer’s experience and diagnostic method (22). Consequently, there are disputes over the sensitivity (and to a lesser extent specificity) of TVS for detecting USL endometriosis. Some studies reported a low sensitivity for TVS to demonstrate DIE in USL (4, 18, 23-25), whereas some others, similar to the current research, concluded that TVS is highly sensitive for the detection of USL endometriosis (22, 26). Generally, anatomic complexities (especially in patients with pelvic adhesion/POD obliteration) and a small lesion size can lead to underdiagnosis. However, clinical awareness, professionally trained operators, and certain diagnostic methods (i.e., tenderness-guided methods and standoff techniques for near-field areas) may increase the detection rate (24, 27).
The ovarian mobility has been reported as the most accurate ultrasound indicator of pelvic adhesions, and ovarian/uterine mobility is acceptable for diagnosing endometriosis (6, 24, 28). This observation confirms the current results, although laparoscopic visualization was more promising in the present study. Transvaginal sonography has been shown to be a promising modality for detecting pelvic adhesions and POD obliteration (28-30), which is consistent with our findings. Additionally, some studies reported a high level of TVS-laparoscopy agreement for ovarian adhesions that are either mobile or fixed on palpation.
Some studies suggested that TRS may improve the detection rate of DIE (23, 31, 32) and can help diagnose endometriosis in the intestines (6, 8, 33, 34). On the other hand, Bazot et al. compared the diagnostic yield of TVS and TRS in patients with pelvic endometriosis and demonstrated that TVS was very accurate in identifying intestinal and bladder endometriosis (23). Generally, tubal blockade and the resulting dilatation (presenting as either hydrosalpinx or hematosalpinx) are common in DIE and contribute to infertility (35). In the present study, a similar detection rate was reported for hydrosalpinx/hematosalpinx on TVS and laparoscopy. Based on the current results, peritoneal cysts were more frequently identifiable on laparoscopy compared to TVS. Likewise, peritoneal cysts were not significantly associated with a higher DIE detection rate on TVS (36).
Considering the high accuracy of TVS for diagnosing DIE in challenging sites, such as distal ureter, bladder base, and upper and lower rectum, which may not be readily accessible during laparoscopy, besides the unique applicability of this modality for the examination of uterine/ovarian motility, it may be even more advantageous for some cases. Additionally, TVS has been shown to be more accurate in identifying DIE lesions in patients with a minimal or mild disease or when lesions are atypical in terms of morphology, although they may appear normal on laparoscopy (36).
Precise diagnosis and mapping of DIE can greatly help with treatment or surgical planning (if necessary), thereby reducing the risk of underestimation and incomplete excision of DIE foci and obviating the need for multiple surgical procedures (since non-excised residual lesions tend to grow overtime and involve the adjacent structures) (37, 38). Accurate DIE mapping may suggest the important role of other specialists when bowel, distal ureter, or bladder involvement is detected. Additionally, with an accurate estimation of the disease extent, clinically relevant DIE deposits are more likely to be localized and excised. Moreover, preoperative DIE mapping enables surgery customization, which may preclude complex adhesiogenic surgeries.
The present study had some limitations. First, a small population for each site of involvement may cause sampling bias. To obtain representative samples for each subgroup, comprehensive studies on larger populations or pooling data from different studies are required. Second, pelvic adhesion assessment can be deemed subjective; however, the current study and some other investigations showed that it is accurate enough to be incorporated into daily clinical practice (18). Third, this study did not include asymptomatic cases of DIE, and the results cannot be generalized to all patients. Collection of relevant data from women undergoing exploring laparoscopy or laparoscopy for any other indication, while paying attention to the common sites of DIE plaque deposition may yield different findings and is encouraged in future investigations. Finally, only TVS-positive cases were included in this study, which might cause selection bias, whereas TVS-negative cases (milder forms of pelvic DIE) who may show DIE on laparoscopy were not included; this can influence the agreement of TVS and laparoscopic findings for some sites, if not all; nevertheless, nodules which are missed on TVS tend to be smaller and easier to excise, with a lower risk of iatrogenic trauma in the bladder, ureters, and bowel wall (27).
In conclusion, the current findings showed that TVS is an accurate and non-invasive tool for detecting and mapping DIE and POD obliteration, regardless of the presence of OE, tubal dilation, or pelvic cysts and adhesions. Transvaginal sonography can be regarded as a useful tool for identifying DIE preoperatively, as it may waive the need for exploratory or confirmatory laparoscopy in DIE or at least facilitate precise pre-procedural DIE mapping, besides the prediction of surgical difficulties, surgery duration, postoperative complications, and length of hospital stay.


.jpg)