1. Background
Esophageal cancer subtypes, including squamous cell carcinoma (SCC) and adenocarcinoma, have dominant characteristics, such as lymphatic spread due to the absence of a serosa layer in the esophagus and risk of systemic metastasis. It is generally essential to determine both local and distant metastatic spread of esophageal cancer for precise treatment planning (1). Metabolic imaging using flourine-18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) scan has received special attention in detecting the local spread of disease and its distant metastasis for initial staging and treatment planning. This technique has been particularly effective in detecting distant metastatic lesions (2, 3) and also in the assessment of response to neoadjuvant therapies, which is fundamental to treatment for many esophageal cancer patients (4, 5). However, modalities, such as computed tomography (CT) scan and endoscopic ultrasound (EUS), are mainly employed for the diagnosis of locally advanced tumors and commonly fail to detect distant metastatic lesions (6).
The predictive potential of 18F-FDG PET/CT scan for the survival of esophageal cancer patients remains unknown following routine treatments (7). To date, 18F-FDG PET/CT scan has been recommended for the initial staging of esophageal cancer patients. It has been demonstrated that 18F-FDG PET/CT findings might be correlated with the tumor grade and aggressiveness (8). According to our literature review, only few studies have evaluated the association of 18F-FDG PET/CT semi-quantitative parameters with local and distant extension of esophageal cancer and patient survival (9, 10).
2. Objectives
This study aimed to evaluate a semi-quantitative parameter of 18F-FDG PET/CT, that is, maximum standardized uptake value (SUVmax), in patients with SCC and adenocarcinoma subtypes and to investigate its ability to differentiate between these two categories. Moreover, this study aimed to evaluate the potential of 18F-FDG PET/CT scan in the assessment of tumor characteristics, such as local and distant metastatic extensions, and to determine the association of tumor SUVmax with patient survival.
3. Patients and Methods
This cross-sectional study was performed on newly diagnosed patients with one of the major subtypes of esophageal carcinoma, that is, SCC or adenocarcinoma, who were referred for initial staging during 2013 - 2019. To the best of our knowledge, there was no confounding risk factor (unrelated to underlying esophageal cancer) in this study affecting the number of patients with either SCC or adenocarcinoma. The patients’ background information, including the demographic data, medical and pharmacological records, clinical symptoms, final diagnostic pathology findings, and follow-up records were collected by reviewing their medical records.
The 18F-FDG-PET/CT images were analyzed on an Advantage Workstation version 4.5 (ADW 4.5), and all images of the patient were reviewed for primary tumor (T), metastatic regional lymph nodes (N), and distant metastatic lesions (M). The semi-quantitative parameter, SUVmax, was calculated automatically for each primary tumor, regional lymph node, and distant metastatic lesion for each patient in a designated region of interest (ROI) for each lesion. The images were reviewed by two readers simultaneously. One experienced nuclear medicine physician and one experienced radiologist with specific PET training reviewed all the images, and the final decision was made based on their consensus. Information regarding the treatment protocol (i.e., surgery, chemotherapy, and radiotherapy) was also extracted by reviewing the patients’ medical records.
The patients were followed-up for 12-24 months after the initial treatment and were reevaluated for restaging with 18F-FDG PET/CT scan. Response to treatment (complete or partial) was defined as a complete or partial reduction of the size or metabolic activity of all TNM staging components on follow-up imaging. Non-response to treatment (stable or progressive disease) was defined as a stable disease or an increase in the size or metabolic activity of one of the TNM components on follow-up imaging. Any new lesion or any increase in the size or metabolic activity of any TNM components represented a progressive disease.
For statistical analysis, data are presented as mean ± standard deviation (SD) for quantitative variables and summarized by frequency (percentage) for categorical variables. Continuous variables were compared using t-test or Mann-Whitney U test if the data did not have a normal distribution or if the assumption of equal variance was violated across the study groups. The categorical variables were also compared using chi-square test. P-values ≤ 0.05 were considered statistically significant. For statistical analysis, SPSS version 23.0 for Windows (IBM SPSS Statistics for Windows, released in 2015, IBM Corp., Armonk, NY, USA) was used.
4. Results
A total of 36 patients with esophageal SCC and 15 patients with esophageal adenocarcinoma were included in this study. Comparison of the two groups regarding the baseline characteristics (i.e., mean age and sex) indicated no significant difference between these groups (Table 1). Regarding the tumor location, SCC was more commonly located in the mid portion of the esophagus, while adenocarcinoma was located prominently in the distal part. Based on the 18F-FDG-PET/CT findings (Table 1), no significant difference was observed between the SCC and adenocarcinoma subtypes in terms of the mean primary tumor SUVmax (12.97 ± 7.74 vs. 10.41 ± 5.48, P = 0.283), lymph node involvement (50.0% vs. 46.7%, P = 0.122), and presence of distant metastasis (25.0% vs. 40.0%, P = 0.325).
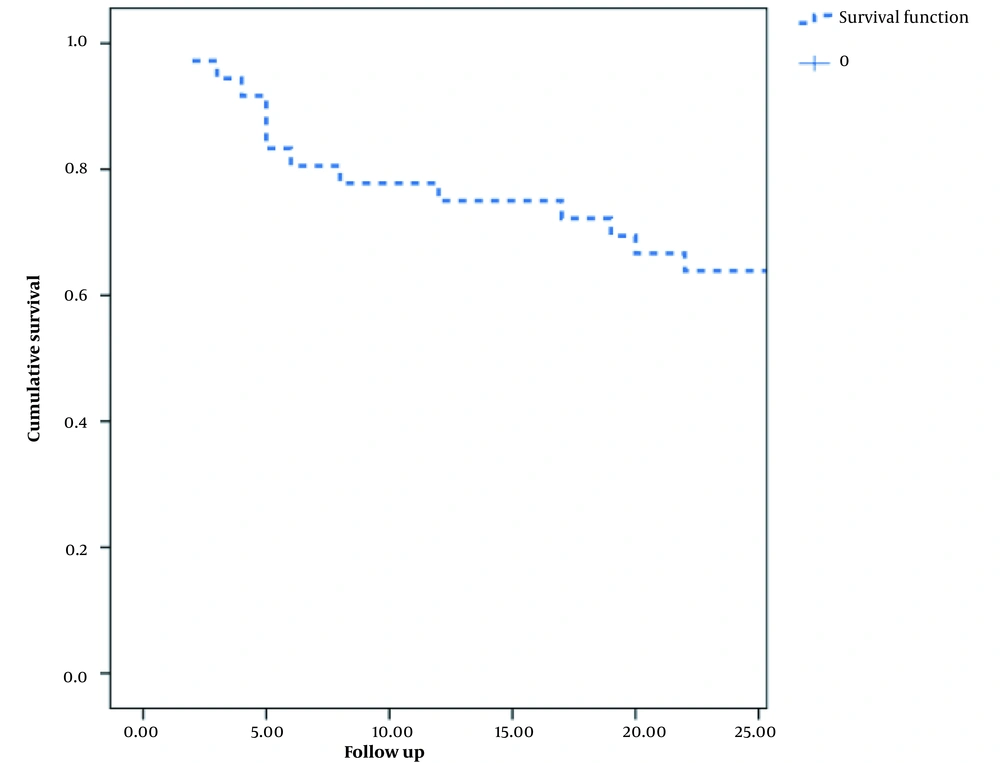
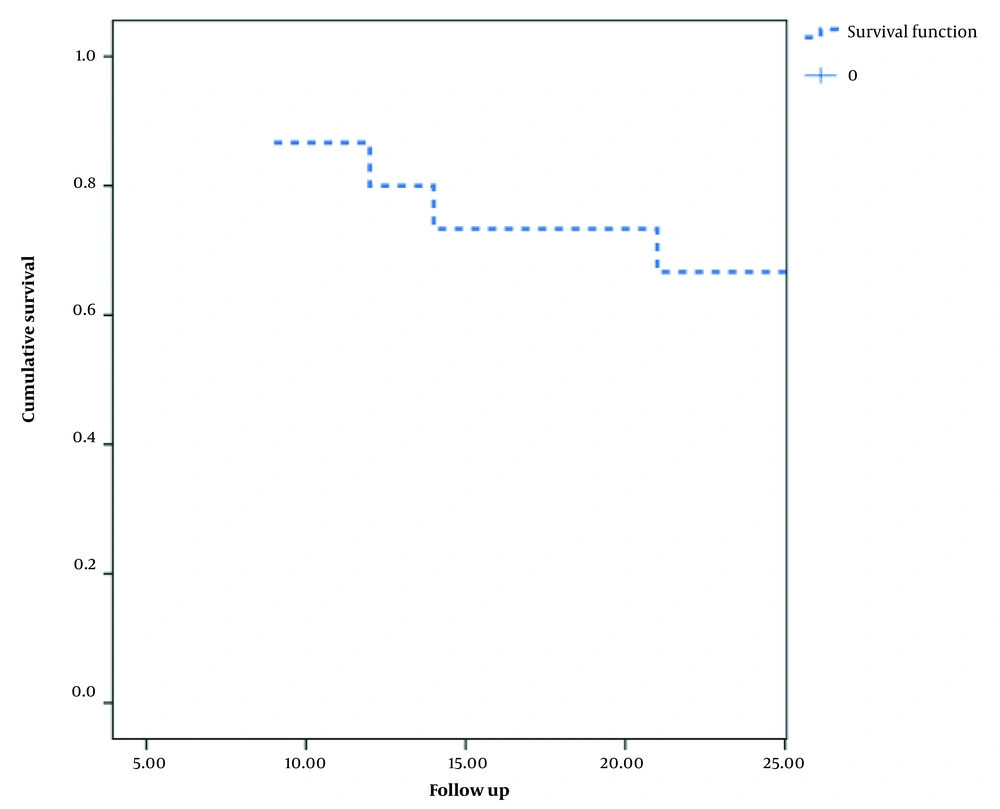
With respect to treatment outcomes, 69.4% of patients with SCC and 53.3% of patients with adenocarcinoma were responsive to neoadjuvant chemoradiotherapy. Surgery was subsequently required for 27.8% of SCC patients and 26.7% of adenocarcinoma patients. Death was reported in 36.1% of SCC patients and 40.0% of adenocarcinoma patients, with no significant difference between the groups (P = 0.103). Overall, one- and two-year survival rates were 75.0% and 63.9% in the SCC group and 80.0% and 60.0% in the adenocarcinoma group, indicating no significant inter-group difference (Table 1).
| Variables | SCC group | Adenocarcinoma group | P-value |
|---|---|---|---|
| Demographic information | |||
| Male | 21 (58.3) | 8 (53.3) | 0.743 |
| Female | 15 (41.7) | 7 (46.7) | 0.436 |
| Mean age (y) | 64.53 ± 12.47 | 60.67 ± 13.16 | 0.326 |
| Tumor location | 0.014 | ||
| Distal | 18 (50.0) | 14 (93.3) | |
| Mid-portion | 15 (41.7) | 0 (0.0) | |
| Proximal | 2 (5.6) | 0 (0.0) | |
| Diffuse | 1 (2.8) | 1 (6.7) | |
| PET/CT findings | |||
| Mean SUVmax of the primary tumor | 12.97 ± 7.74 | 10.41 ± 5.48 | 0.283 |
| Mean SUVmax of metastatic lymph nodes | 5.58 ± 3.51 | 8.47 ± 5.34 | 0.130 |
| Mean SUVmax of distant metastatic lesions | 5.65 ± 3.16 | 5.00 ± 4.82 | 0.789 |
| Lymph node involvement present | 18 (50.0) | 7 (46.7) | 0.122 |
| Distant metastasis present | 9 (25.0) | 6 (40.0) | 0.325 |
| Treatment outcome | |||
| Responder to treatment | 25 (67.5) | 8 (53.3) | 0.273 |
| Non-responder to treatment | 12 (32.5) | 7(46.7) | 0.079 |
| Surgery required following neoadjuvant therapy | 10 (27.8) | 4 (26.7) | 0.935 |
| Death during follow-up | 13 (36.1) | 6 (40.0) | 0.794 |
| Mean interval between diagnosis and death (months) | 9.85 ± 7.19 | 17.00 ± 10.75 | 0.124 |
| One-year survival | 75.0% | 80.0% | 0.562 |
| Two-year survival | 63.9% | 60.0% | 0.437 |
Abbreviations: SCC, squamous cell carcinoma; PET/CT, positron emission tomography/computed tomography; SUV, standardized uptake value.
a Values are expressed as mean ± SD or No. (%).
b There is no statistically significant finding.
According to Table 2, comparison of responder and non-responder subgroups showed no significant difference in the mean SUVmax values of the primary tumor, lymph nodes, or distant metastasis between these subgroups of SCC and adenocarcinoma groups.
| Variables | Response (+) | Response (-) | P-value |
|---|---|---|---|
| SCC type | |||
| Mean SUVmax of primary tumor | 12.78 ± 7.93 | 13.34 ± 7.73 | 0.852 |
| Mean SUVmax of metastatic lymph nodes | 4.49 ± 2.10 | 7.14 ± 4.62 | 0.129 |
| Mean SUVmax of distant metastasis | 3.46 ± 2.82 | 7.30 ± 2.51 | 0.127 |
| Adenocarcinoma type | |||
| Mean SUVmax of primary tumor | 10.06 ± 6.32 | 10.82 ± 4.88 | 0.816 |
| Mean SUVmax of metastatic lymph nodes | 10.66 ± 8.10 | 6.82 ± 2.22 | 0.395 |
| Mean SUVmax of distant metastasis | 2.70 ± 1.22 | 5.76 ± 5.60 | 0.683 |
Abbreviations: SCC, squamous cell carcinoma; SUV, standardized uptake value; 18F-FDG PET/CT, Flourine-18 fluorodeoxyglucose positron emission tomography/computed tomography.
a There is no statistically significant finding.
As demonstrated in Table 3, the SUVmax of distant metastasis was significantly higher in deceased patients compared to survivors, with no significant difference in the SUVmax of primary tumor or lymph nodes in the SCC group (Table 3). In patients with adenocarcinoma, the SUVmax values of the primary tumor, lymph nodes, and distant metastasis were not significantly different between the deceased and surviving patients and were not predictive of patient survival.
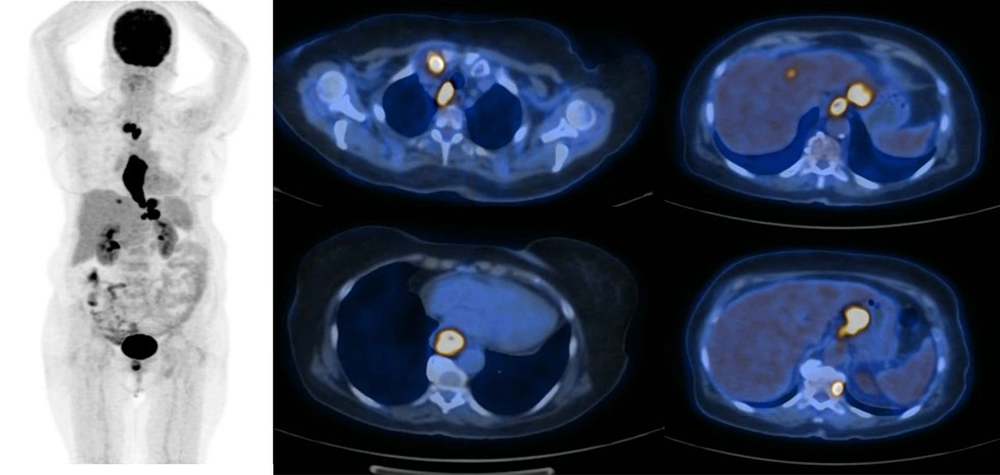
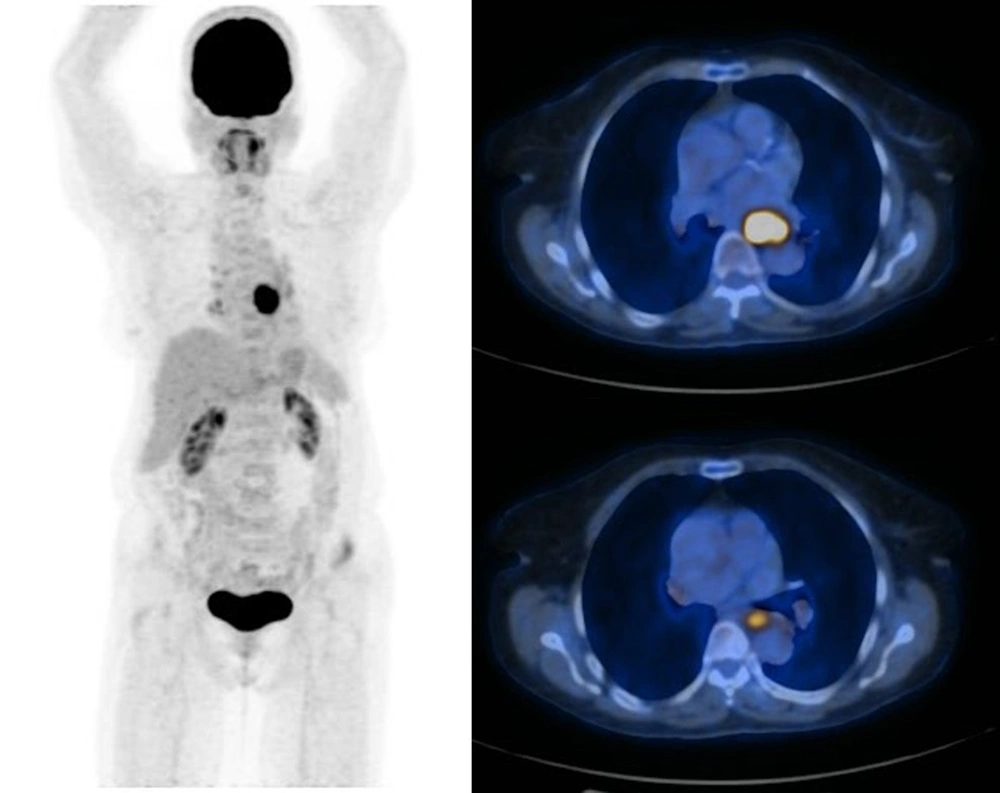
Figures 1 and 2 demonstrate the Kaplan-Meier curves for the two-year survival of patients with esophageal SCC and adenocarcinoma, respectively. Also, Figures 3 and 4 demonstrate patients with esophageal SCC and adenocarcinoma, respectively.
| Index | Death (+) | Death (-) | P-value |
|---|---|---|---|
| SCC type | |||
| Mean SUVmax of primary tumor | 12.39 ± 6.52 | 13.37 ± 8.63 | 0.731 |
| Mean SUVmax metastatic lymph node | 6.38 ± 4.59 | 4.86 ± 2.21 | 0.390 |
| Mean SUVmax of distant metastasis | 7.18 ± 2.18 | 1.85 ± 0.49 | 0.023 a |
| Adenocarcinoma type | |||
| Mean SUVmax of primary tumor | 10.81 ± 4.88 | 10.06 ± 6.32 | 0.816 |
| Mean SUVmax of metastatic lymph nodes | 6.82 ± 2.22 | 10.66 ± 8.09 | 0.395 |
| Mean SUVmax of distant metastasis | 5.76 ± 5.60 | 2.70 ± 1.68 | 0.683 |
Abbreviations: SCC, squamous cell carcinoma; SUV, standardized uptake value; PET/CT, positron emission tomography/computed tomography.
a Statistically significant finding.
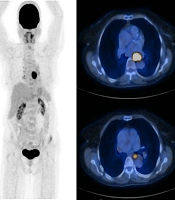
The maximum intensity projection (MIP) and axial PET/CT images of a patient with esophageal adenocarcinoma. There is a focal mid-esophageal primary tumor and no definite evidence of hypermetabolic distant metastasis. Mildly hypermetabolic lymph nodes were reactive in the final pathology report.
5. Discussion
The role of 18F-FDG PET/CT scan in the initial staging of esophageal carcinoma has been extensively studied (11). Although according to the National Comprehensive Cancer Network (NCCN) guidelines (12), there is not enough evidence to justify the routine application of this imaging modality for the initial staging of all esophageal carcinoma patients, it has been recommended by several associations, including the American Association for Thoracic Surgery (AATS) (13). The AATS emphasized the importance of 18F-FDG PET/CT scan in detecting distant metastatic sites, which is essential in treatment planning. Besides, few other studies have evaluated the prognostic value of 18F-FDG PET/CT scan in esophageal cancer patients and reported contradictory results (14).
The present study evaluated the potential of 18F-FDG PET/CT scan in predicting response to treatment and prognosis of patients with esophageal SCC and adenocarcinoma. One significant finding of this study was that measurement of SUVmax in distant metastatic lesions was significantly associated with mortality in the SCC group, whereas no such association was observed in patients with adenocarcinoma. Nevertheless, the results of previous studies regarding the prognosis of patients with esophageal cancer based on 18F-FDG PET/CT findings are contradictory.
In a study by Mantziari et al. on patients with SCC, high values of SUVmax had a significant relationship with higher tumor stages and could predict tumor recurrence and long-term survival (15). In another study by Kim et al., the SUVmax of primary esophageal tumor lesion could predict distant metastasis with 67% sensitivity, 83% specificity, and 76% diagnostic accuracy (16). In a study by Bosch et al. on patients with esophageal adenocarcinoma, the tumor SUVmax predicted local tumor progression with 40% sensitivity and 73% specificity (17), which contradicted the findings of the current study. However, in a study by Fatima et al., similar to the present research, there was no significant difference in the SUVmax values of the primary tumor and metastatic regional lymph nodes between the SCC and adenocarcinoma groups (18).
Additionally, in a study by Lindner et al., the value of SUVmax was significantly related to tumor stage and tumor size. If patients had SUVmax values < 6 at the primary tumor site, surgery resulted in a higher long-term survival compared to cases with SUVmax values > 6; nevertheless, no such relationship was detected in response to neoadjuvant therapy (19). In another study by Wang et al. to determine the stage of esophageal tumor, the diagnostic accuracy of 18F-FDG PET/CT scan was 85.7%. There was also a significant difference in the value of SUVmax between the T2 and T3 groups (20). Overall, 18F-FDG PET/CT scan showed high potential in predicting the outcomes and short-term survival of patients with esophageal SCC compared to esophageal adenocarcinoma.
It should be noted that the results of the present study might be potentially affected by the small sample size; therefore, further studies with a larger sample size and a longer follow-up are recommended.
In conclusion, the SUVmax value according to 18F-FDG PET/CT scan for distant metastatic lesions could predict the short-term survival of patients with SCC. However, in patients with esophageal adenocarcinoma, the value of SUVmax on 18F-FDG PET/CT scan was not predictive of patient survival.
.jpg)