1. Background
Worldwide, lung cancer is not only the most common tumor, but also the tumor of supreme incidence and death rate in all malignant tumors (1). Bronchoscopy has been widely used in the diagnosis of space occupying lung lesions, but the diagnostic rate of pulmonary peripheral lesions (PPL) is relatively low (2, 3). PPL refers to the pulmonary peripheral lesions, which were unreachable by bronchoscope, under the 6th order segmental bronchi. Although the diagnostic rate for PPL by bronchoscopy increased with the help of X-ray fluoroscopy (4), the exposure to radiation still limited the clinical application, while the intravascular ultrasound technology overcame the limitations and was a milestone in the development of respiratory endoscopy. The development of miniature lung ultrasound probe has extremely improved the diagnostic accuracy of PPL, especially peripheral lung cancer (5-10). This study retrospectively analyzed the diagnostic rate of endobronchial ultrasound guided sheath (EBUS-GS) guided trans-bronchial lung biopsy (TBLB) in PPL, as well as the complications of the diagnostic procedure, which aimed to define the value of EBUS-GS guided TBLB in PPL diagnose (11, 12).
2. Objectives
This study retrospectively analyzed the diagnostic rates, influencing factors, and operation time of TBLB guided by conventional CT, EBUS-GS, and endobronchial ultrasound guided sheath with virtual bronchoscopy, to investigate the diagnostic value of EBUS-GS+VB-TBLB for PPL, and to determine the correlative factors that are related to greater diagnostic accuracy of EBUS-GS-TBLB.
3. Patients and Methods
3.1. Subjects
The clinical data of outpatients and inpatients who underwent continuous treatment in the Bronchoscopy Center of the Second Hospital of Shandong University from January 2015 to May 2016 were retrospectively analyzed. In all patients, chest CT examination revealed PPLs, which were surrounded by the lung parenchyma. The PPLs were not found with conventional bronchoscopy (Olympus BF-260), and there were no airway lesions, structural abnormalities, or mucosal lesions. The patients required TBLB, and having no bronchoscopic contraindications, were enrolled according to their time of admission. Based on the operation, the patients were assigned to the conventional group, the E-TBLB group, or the E +VB-TBLB group.
3.2. CT Value Assessment
The anatomical locations of the lesions were preoperatively determined by CT. The initial location and CT attenuation value of the lesion were determined by CT imaging before the operation. All CT images were collected using a 64-slice spiral CT and we defined the CT level as the maximum section of the lesion, avoiding vascular necrosis, the bronchial cavity, and other factors that would significantly interfere with the CT radiodensity value. We recorded the minimum diameter of the lesion for calculating the radius of a circle to determine the CT attenuation value. The average of the measurements taken by four senior doctors was used.
3.3. Fiberoptic Bronchoscope Operation
The patients, who had fasted 6 hours before surgery, received 2% lidocaine by inhalation, and were monitored for heart rate, peripheral oxygen saturation, and blood pressure during surgery. A BF-1T260 or BF-1T240 (BF-P260F) flexible bronchoscope (Olympus, Japan) was used together with an endoscopic ultrasound system (EU-M30S, EU-ME1, Olympus, Japan), a 2.0 (1.8) mm 30 (20) MHz intracavitary ultrasound probe (UM-S30-20R, Olympus, Japan), and a guide sheath kit (K-203 [K-201], Olympus, Japan). (1) Group TBLB: according to the lesion location, determined preoperatively by chest CT, the biopsy forceps was slowly advanced into the corresponding sub-bronchus, withdrawn 3 cm when it met with resistance, then opened and advanced 2 cm for sampling the specimen, which was done at the end of expiration. (2) Group E-TBLB: the biopsy forceps and cell brush were placed into the guide sheath before surgery according to the operating instructions and the position was marked; the EBUS probe was then placed and fixed in the guide sheath. When the bronchoscope reached the lesion location as determined preoperatively, the EBUS probe, wrapped by the sheath, was introduced via the bronchoscope channel, and moved gradually to approach the lesion location to obtain the EBUS image. During the procedure, the probe and guide sheath were continuously adjusted according to the EBUS image to ensure the lesion completely surrounded the probe. When an appropriate EBUS image was obtained, the bronchoscope was fixed in the nasal cavity, with the external part of the guide sheath being fixed at the entrance of the bronchoscope channel. The probe was then withdrawn while the sheath was left in position. The biopsy forceps or cell brush was then guided in for sampling. (3) Group E +VB-TBLB: the patient’s HRCT was imported into the virtual bronchoscopic navigation software (Direct Path, Olympus, Japan) in the DICOM format to mark the lesion and determine the optimal biopsy path (the software can automatically generate and select the best navigation path to guide the EBUS probe toward the target lesion). The rest of the operation was the same as for group E-TBLB. All groups were sampled for five specimens, which were fixed in a 4% formaldehyde solution and prepared as smears for pathological examination. Lesion diameter was determined as 1/2 of the sum of the longest and shortest diameters. All of our punctures were multi-point punctures, with more than five punctures and we would do on-site cytological examinations to make sure the pathological tissue samples we provided were reliable and effective. There were three respiratory doctors and one cytology laboratory technician and two graduate students.
3.4. Operation Duration
Time was started when the fiberoptic bronchoscope passed through the glottis into the trachea, and ended when the fiberoptic bronchoscope passed through the glottis out of the trachea.
3.5. Determination of Pathology
If the lesion image or biopsy revealed no pathology or the biopsy lesion was identified as benign, then CT-guided lung puncture, post-treatment follow-up for a year, or surgery was performed according to the patient’s choice. False negative was one of the most severe limitations of TBLB, although multi-point puncture was conducted, so we would follow up these patients for one year to observe the treatment effect. All pathologic results were determined by two experienced pathologists, and if the results were inconsistent, a third pathologist was consulted to make a final decision.
3.6. Statistical Analysis
SPSS version 24.0 (IBM SPSS Statistics, version 24.0. Armonk, NY: IBM Corp) software was used for the statistical analysis. The continuous data in this study followed a normal distribution, so they were expressed as means ± standard deviation. An analysis of variance was used for the intergroup comparison. The intergroup comparison of disordered-classified data was analyzed with the χ2 test, with P < 0.05 considered statistically significant.
4. Results
4.1. General Data
A total of 87 patients were included in the study, with 38 patients in group TBLB, including 28 men and 10 women aged 27 - 78 years, with a mean of 59.6 ± 9.9 years, and average lesion size of 21.6 ± 6.3 mm. Group E-TBLB consisted of 29 patients, including 20 men and nine women aged 29 - 76 years, with a mean of 57.0 ± 11.3 years, and average lesion size of 20.4 ± 6.1 mm. Group E+V-TBLB had 20 patients, including 14 men and six women aged 31 - 79 years, with a mean of 60.7 ± 10.4 years, and average lesion size of 20.3 ± 4.8 mm. There was no significant difference in sex, age, and lesion size among the three groups (P > 0.05) (Table 1).
| Item | TBLB (n = 38) | E-TBLB (n = 29) | E+V-TBLB (n = 20) | χ2/t | P value |
|---|---|---|---|---|---|
| Gender, M/F | 28/10 | 20/9 | 14/6 | 0.199 | 0.905 |
| Age, y | 59.6 ± 9.9 | 57.0 ± 11.3 | 60.7 ± 10.4 | 0.867 | 0.424 |
| Lesion size, mm | 21.6 ± 6.3 | 20.4 ± 6.1 | 20.3 ± 4.8 | 0.415 | 0.661 |
Abbreviations: TBLB, trans-bronchial lung biopsy; E-TBLB, endo-trans-bronchial lung biopsy; E+V-TBLB, endobronchial ultrasonography with a guide sheath combined with virtual bronchoscopic biopsy
aValues are expressed as mean ± SD.
4.2. Diagnostic Rate
The diagnostic accuracy in groups TBLB, E-TBLB, and E+V-TBLB were 47.4% (18/38), 75.9% (22/29), and 80% (16/20), respectively. The accuracy rate in group TBLB was significantly lower than that in the other two groups (χ2 = 8.589, P = 0.014), but there was no significant difference between groups E-TBLB and E+V-TBLB (P > 0.05). Different pathological diagnoses of lesions and distribution of anatomical location have been mentioned in Tables 2 and 3.
| Pathological diagnosis | TBLB | E-TBLB | E+V-TBLB |
|---|---|---|---|
| Hamartoma | 2 (5.3) | 1 (3.4) | 1 (5.0) |
| Large cell carcinoma | 1 (2.6) | 0 (0.0) | 1 (5.0) |
| Pneumonia | 5 (13.2) | 1 (3.4) | 0 (0.0) |
| Tuberculosis | 3 (7.9) | 1 (3.4) | 2 (10.0) |
| Sarcoidosis | 1 (2.6) | 0 (0.0) | 1 (5.0) |
| Squamous cell carcinoma | 2 (5.3) | 3 (10.4) | 2 (10.0) |
| Granuloma | 1 (2.6) | 1 (3.4) | 0 (0.0) |
| Adenocarcinoma | 14 (36.8) | 11 (38.0) | 9 (45.0) |
| Small cell carcinoma | 1 (2.6) | 3 (10.4) | 1 (5.0) |
| Inflammatory pseudotumor | 3 (7.9) | 3 (10.4) | 1 (5.0) |
| Inflammation | 0 (0.0) | 0 (0.0) | 1 (5.0) |
| Carcinoma in situ | 3 (7.9) | 1 (3.4) | 0 (0.0) |
| Metastatic tumor | 2 (5.3) | 4 (13.8) | 1 (5.0) |
| Total | 38 | 29 | 20 |
Abbreviations: TBLB, trans bronchial lung biopsy; E-TBLB, endo-trans bronchial lung biopsy; E+V-TBLB, endobronchial ultrasonography with a guide sheath combined with virtual bronchoscopic biopsy
| Pathological location | TBLB | E-TBLB | E+V-TBLB |
|---|---|---|---|
| Right upper lobe | 4 (10.5) | 7 (24.1) | 2 (10.0) |
| Right middle lobe | 9 (23.7) | 9 (31.1) | 3 (15.0) |
| Right lower lobe | 5 (13.2) | 3 (10.3) | 4 (20.0) |
| Left upper lobe | 6 (15.8) | 2 (6.9) | 3 (15.0) |
| Left lingual lobe | 4 (10.5) | 2 (6.9) | 3 (15.0) |
| Left lower lobe | 10 (26.3) | 6 (20.7) | 5 (25.0) |
Abbreviations: TBLB, trans-bronchial lung biopsy; E-TBLB, endo-trans-bronchial lung biopsy; E+V-TBLB, endobronchial ultrasonography with a guide sheath combined with virtual bronchoscopic biopsy
4.3. Operation Time
Since the data were not normally distributed, the median (Q25, Q75) was used. The average operation time in group was 9.71 min (8.35, 13.40), 12.25 minutes (1.06, 15.81), and 7.34 minutes (6.35, 8.22) respectively in groups TBLB, E-TBLB, and E+V-TBLB. Then Kruskal Wallis rank sum test was used. The chi-square value was 29.139 (P < 0.001).
4.4. Complications
All the patients tolerated the TBLB operation well. Bleeding of greater than 20 mL occurred in one patient in group TBLB, which was noted as blood in the drainage bottle, but the symptoms improved after treatment. Groups E-TBLB and E+V-TBLB exhibited a small amount of bleeding, visible under microscope. No patients from any group had pneumothorax, hemoptysis, or other complications.
4.5. Analysis of TBLB Positive Rate-Related Factors
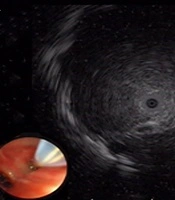
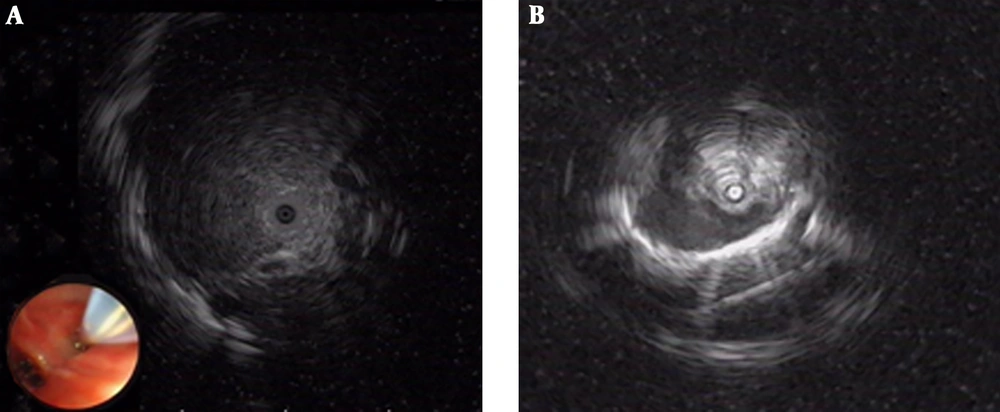
Among the 49 patients in groups E-TBLB and E+V-TBLB, 29 had a lesion diameter ≥ 2 cm, in which 27 patients were diagnosed by EBUS-GS-TBLB, with a diagnosis rate of 93.1% (27/29). Of the 20 patients that had a lesion diameter ≤ 2 cm, 11 were diagnosed by EBUS-GS-TBLB, with a diagnosis rate of 55% (11/20). The difference in diagnosis rate was statistically significant (Table 4, χ2 = 7.804, P = 0.005). Among the 32 patients with probe-penetrable lesions, 30 patients were diagnosed by EBUS-GS-TBLB, with a diagnosis rate of 93.5% (30/32), while among the 17 patients with non-probe-penetrable lesions, eight patients were diagnosed by EBUS-GS-TBLB, with a diagnosis rate of 47.1% (8/17). This difference in diagnosis rate was also statistically significant (χ2 = 11.350, P = 0.001) (Table 4 and Figure 1). The diagnostic rate for the left superior lobe was lower than that for the other lobes, but the difference was not statistically significant (χ2 = 5.827 P = 0.296) (Table 4); and the location of the lesions have been presented in Table 3. Diagnostic accuracy of CT in groups E-TBLB and E+V-TBLB when HU was considered, were 92.3% and 100% respectively in HU higher than 20, 100% and 66.7% respectively in HU between -200 and 20 and 33.3% and 70% respectively when HU was lower than -200 HU. The diagnostic accuracy of the CT was lowest at HU lower than -200 (χ2 = 10.196, P < 0.01) (Table 4).
| EBUS-GS group (E-TBLB and E+V-TBLB groups together) | E -TBLB group | E+V-TBLB group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Diagnostic rate (%) (positive/total) | χ2 | P value | Diagnostic rate (%) (positive/total) | χ2 | P value | Diagnostic rate (%) (positive/total) | χ2 | P value | |
| Lesion diametera | 7.8 | 0.005 | 7.5 | 0.006 | * | 0.255 | |||
| ≥ 2 cm | 93.1 (27/29) | 94.1 (16/17) | 91.7 (11/12) | ||||||
| < 2 cm | 55.0 (11/20) | 50.0 (6/12) | 62.5 (5/8) | ||||||
| Lesion distribution | 5.8 | 0.296 | 2.7 | 0.747 | 6.2 | 0.187 | |||
| Right upper lobe | 66.7 (6/9) | 71.4 (5/7) | 50.0 (1/2) | ||||||
| Right lower lobe | 91.7 (11/12) | 88.9 (8/9) | 100.0 (3/3) | ||||||
| Right middle lobe | 85.7 (6/7) | 66.7 (2/3) | 100.0 (4/4) | ||||||
| Left upper lobe | 40.4 (2/5) | 50.0 (1/2) | 33.3 (1/3) | ||||||
| Left lingual lobe | 80.0 (4/5) | 50.0 (1/2) | 100.0 (3/3) | ||||||
| Left lower lobe | 81.8 (9/11) | 83.3 (5/6) | 80.0 (4/5) | ||||||
| Penetration of ultrasonic probe go through the lesiona | 11.4 | 0.001 | 5.6 | 0.018 | * | 0.007 | |||
| Can | 93.8 (30/32) | 89.4 (17/19) | 100.0 (13/13) | ||||||
| Can’t | 47.1 (8/17) | 50.0 (5/10) | 42.9 (3/7) | ||||||
| CT values | 10.2 | 0.005 | 10.9 | 0.002 | 2.8 | 0.31 | |||
| < -200 Hu | 52.6 (10/19) | 33.3 (3/9) | 70 (7/10) | ||||||
| -200 - 20 Hu | 90.0 (9/10) | 100 (7/7) | 66.7 (2/3) | ||||||
| > 20 Hu | 95.0 (19/20) | 92.3 (12/13) | 100 (7/7) | ||||||
Abbreviations: TBLB, trans-bronchial lung biopsy; E-TBLB, endo-trans-bronchial lung biopsy; E+V-TBLB, endobronchial ultrasonography with a guide sheath combined with virtual bronchoscopic biopsy
aCalculated by Fisher's exact test.
5. Discussion
Lung cancer is currently the malignant tumor with the highest incidence rate worldwide, and early diagnosis is extremely important for improving prognosis. Lesions located in the large airways often can be diagnosed by bronchoscopy, but those in smaller peripheral airways are often difficult to explore with conventional bronchoscopy due to the presence of stenotic interlinked bronchi. TBLB lung biopsy based on CT imaging has disadvantages, such as low detection rates, hemorrhage, and a high risk of pneumothorax. CT-guided transthoracic fine needle aspiration (TFNA) has a higher diagnostic accuracy than does TBLB, but its safety has been questioned due to its risk of hemorrhage, pneumothorax, or needle-way graft translocation (13).
The clinical application of EBUS enables PPLs to be “visualized”, thus greatly improving the accuracy and precision of biopsy, reducing the blind approach of conventional biopsy, and significantly improving the positive diagnostic rate of PPLs (14-16). The ultrasound probe can be placed directly in the airway lesions via the fiberoptic bronchoscopic channel, so it shortens the propagation path of the sound wave and reduces its attenuation. In addition, it uses high frequency technology to improve image resolution and to micronize the images of tissue structures, enabling acoustic stratification near the trachea and bronchial walls, which permits “microstructure scanning”. Since the EBUS probe can image the bronchial wall and its adjacent tissue structures (within 4 cm, including the mediastinum), the endoscopic field from the airway cavity to the outside wall and from the large airways out to the smaller airways (about 2 mm in diameter) is greater, which expands the visual and work fields of the endoscopists, and greatly improves the diagnostic level of the ultrasound (17). In addition, the imaging performance of the ultrasonic probe enables the operator to determine the blood supply and blood vessel size of the lesion, and to calculate the distance between the lesion vessel and the lumen, permitting a biopsy that avoids sites rich in blood vessels through the identification of these abnormal locations in the sonogram. This not only reduces the incidence of bleeding during biopsy but also significantly improves the operator’s ability to predict bleeding complications, thus greatly improving safety and accuracy of PPL biopsy. In 2004, for the first time, Kurimoto reported that the EBUS-GS technique could improve the diagnostic certainty of PPLs (14).
This study retrospectively analyzed the clinical data of 87 PPL patients who underwent TBLB in the Second Hospital of Shandong University from January 2015 to March 2016, including 38 cases of conventional TBLB, 29 cases of EBUS-GS-TBLB, and 20 cases of endobronchial ultrasound- guide sheath with virtual bronchoscopic biopsy -transbronchial needle aspiration (EBUS-GS-TBNA). The results showed that the positive diagnostic rate in group TBLB was 47.4%, which was far lower than that in group E-TBLB (75.9%), and group E+V-TBLB (80%), and this result was consistent with those from other international studies (18).
The diagnostic rates of EBUS-GS in previous studies ranged from 46% to 86.2% (19-22). Therefore, determining how to improve the diagnostic rate of EBUS-GS for PPL is of great interest worldwide. In this study, we analyzed the lesion size, lesion distribution, CT attenuation value, and relationship between the lesion and the ultrasound probe among the 49 patients in groups EBUS-GS-TBLB and EBUS-GS+VB-TBLB. The positive diagnostic rate for lesions with a diameter less than 2 cm (55.0%) was significantly lower than that for those with a diameter greater than 2 cm (93.1%), consistent with previous reports (14). However, EBUS-GS+VB-TBLB group data, there were no differences between the lesion with a diameter less than 2 cm and greater than 2 cm (P = 0.255), which may based on the accuracy of virtual navigation guidance. Another reason may be the small sample size. The lesion distribution in different lobes did not have an effect on the diagnostic rate. Previous studies have reported that the diagnostic rate of EBUS-GS-TBLB in the two superior lobes is lower. For example, Kurimoto et al. (14) reported the diagnostic rate for the left upper lobe as 40%, significantly lower than that for other lobes (76%). The diagnostic rate for the left upper lobe in this study was also lower than that for other lobes, which is consistent with previous reports. These results may be explained by the large angle that exists between the bronchial opening and the upper level bronchus, which makes it difficult to access the optimal lesion position with the ultrasound probe. This can create the appearance of an abnormal sonogram, and cause the biopsy location to be located just outside the lesion edge, thus making the pathological results appear atypical. The results of this study showed that the diagnostic rate for the lesions that surrounded the ultrasound probe was higher than that for those with their edges adjacent to the probe, which is consistent with other studies (23, 24). The diagnostic accuracy has been reported to be much greater for solid lesions than for frosted glass lesions with EBUS-GS-TBLB (11, 25) but no CT attenuation values were defined as they were in this study, where a CT attenuation value > 20 HU meant greater diagnostic accuracy than a CT attenuation value < -200 HU. The positive diagnostic rate of EBUS-GS TBLB was higher for paracentral lesions with a diameter > 20 mm and CT attenuation value > 20 HU, and with the lesion sampled at a site that completely surrounded the probe. There have been studies that focused on the size and location of the lesion to improve the accuracy of EBUS-GS-TBLB. Some of these studies were guided by virtual bronchoscopy (26), others used a computed tomography workstation (Ziostation, Ziosoft, Tokyo, Japan) (27), and some utilized radiographic fluoroscopic guidance (28), but all required extra technology and instruments, which are not available to many primary hospitals. This study found that for CT attenuation values > 20 HU, EBUS-GS-TBNA was more helpful, while for CT attenuation values < -200 HU, thoracoscopic surgery was recommended, which has a higher diagnostic accuracy and less need for additional medical resources.
The virtual navigation technique is a recently developed technology. The image data is obtained by preoperative high-resolution, thin-layer interval-free chest CT scans are imported into the virtual navigation software system for 3D reconstruction. The software uses pseudo-colors to represent the inner bronchial surface and simulates the bronchial cavity, creating dynamically reconstructed images similar to the actual bronchial cavity. Preoperative chest CT images can be obtained to calibrate the lung lesions and develop the bronchoscopic guide path. Presently, the virtual navigation technique can be used to observe 0 - 6th order bronchi (7). Ishida found that EBUS-GS+VB had higher diagnostic rates than EBUS+VB. While we found no difference between these two techniques for CT attenuation values > 20 HU, this may be related to the guide sheath that we used. After the probe reached the lesion site, we fixed the guide sheath to the site for the biopsy, which should greatly reduce biopsy failure caused by location movement. In the lesions where CT attenuation values were < -200 HU, the diagnostic accuracy was much better when combined with VB since it can be difficult to image using ultrasound under these conditions, whereas the VB technique could guide the probe into the lesion more precisely. Additionally, compared with the VB technique, the radial ultrasound technique alone requires a wider range to detect the bronchial segment in which the lesion is located, so the operation time in group E+V-TBLB was significantly lower than that in group E-TBLB. This also confirms that with the VB technique, the target bronchus could be reached more quickly and accurately, thus helping to improve patients’ comfort and reducing operation risks.
Only one patient in group TBLB exhibited bleeding of more than 20 ml, which was related to the abundant vascularity of the lesion and biopsy-induced injury. For groups EBUS-GS and EBUS-GS+VB, the blood supply and blood vessel conditions were made clear before biopsy by ultrasound, so appropriate pre-biopsy preparations were made, and together with the administration of intravenous and local hemostatic drugs, bleeding was greatly reduced. As a result, no bleeding, pneumothorax, postoperative infection, or other complications occurred in group EBUS-GS and EBUS-GS+VB.
In conclusion, EBUS-GS TBLB is safe and effective in diagnosing PPLs with less trauma, a higher diagnostic rate, and fewer complications. For those lesions with CT attenuation values < -200 HU, EBUS-GS combined with VB can improve the diagnostic accuracy, reduce the operation time, and improve patient tolerance. Selecting PPL cases where the lesion diameter is greater than or equal to 2 cm and the CT attenuation value is > 20 HU, and where the probe can be located in the lesion center, can greatly improve the positive diagnostic rate of EBUS-GS TBLB. As a retrospective study in the real world, it was difficult to randomize the patients, so there would be selection bias and more RCT was needed. This study was a retrospective study and there were selection bias and retrospective bias, lack of data and no randomized control. For the shortcomings of this study, prospective research is further needed.