1. Background
Irritable bowel syndrome (IBS) is one of the common gastrointestinal disorders in clinical practice. Its main clinical features are recurrent abdominal pain with changes in bowel habits, but there are no morphological and biochemical abnormalities that can explain it (1). IBS is a chronic visceral pain disorder associated with severe psychological and mental disorders, such as anxiety, depression and the occurrence of suicidal ideation, which severely impairs the patient’s quality of life (2). Studies showed that anxiety and depression symptoms in IBS patients can affect the processing of visceral stimuli in the central nervous system, leading to further amplification of central pain (3-5). With the recognition of the important role of brain-gut axis in the pathogenesis of IBS, the treatment has shifted from the traditional therapy focusing on the terminal target organ (intestinal tract) to the comprehensive treatment including psychobehavioral therapy to adjust the homeostasis of the brain-gut axis. Combined anxiolytic/antidepressant medications can improve the symptoms of mood disorders and effectively relieve abdominal pain in IBS patients, which is better than other conventional treatments, including placebo, spasmodic and antidiarrheal drugs (6-8).
Flupentixol-melitracen is a combination preparation of has both anxiolytic and antidepressant properties and has been proven to be safe and effective in the treatment of IBS (9, 10). Flupentixol acts on dopamine receptors in the presynaptic membrane, and increasing the amount of dopamine in the synaptic gap to play an antianxiety and antidepressant role. Small doses of melitracen can inhibit the reuptake of norepinephrine and serotonin in presynaptic membrane, and increase the content of monoamine transmitters in the synaptic space, thereby exerting an antidepressant effect. These two components work synergistically and the side effects cancel each other, so as to effectively and timely improve the neuropsychiatric symptoms, regulate the function of the gastrointestinal autonomic nerves, and reduce visceral hypersensitivity, and finally relieve the physical and mental discomfort.
Rectal stimulation task related functional magnetic resonance imaging (fMRI) study confirmed that tricyclic antidepressant reduces activation of some brain regions during pain which were associated with emotional and cognitive functions (11). Resting state fMRI can be used to detect the activity of brain neurons in resting state, and it is an effective tool to evaluate the central mechanism of therapeutic efficacy (12). The method of regional homogeneity (ReHo) measures the synchronization between time series of a specified voxel and its nearest voxel, thus providing an effective tool to characterize neural activity in resting state at the local level (13). Prior studies of our research group found that IBS patients have ReHo changes in the prefrontal-limbic cortex in resting state (14). To our knowledge, this is the first study to investigate the effects of anxiolytic/antidepressant (flupentixol-melitracen) treatment on regional brain function of IBS patients as indexed by ReHo.
2. Objectives
In this study, we hypothesized that combined anxiolytic-antidepressant (flupentixol-melitracen) treatment could reverse abnormal neural activity in the brain regions in IBS patients. Therefore, the purpose of this study was to evaluate the effects of anxiolytic-antidepressant on resting-state brain activity in IBS patients.
3. Patients and Methods
3.1. Study Population
This trial was supported by the Social Development Project of Zhejiang Public Welfare Technology Research (no.: 2015C33292). The research protocol has been approved by the Ethics Committee of the Affiliated Hospital of Hangzhou Normal University (approval no.: HZNU20160427). Informed written consent was obtained from all participants before their enrollment in this study. Thirty-two native right-handed IBS patients were recruited from the digestive disease clinic of our hospital between June 2016 and May 2018. The diagnosis of IBS for each patient was made by two gastroenterologists based on the Rome III symptom criteria (15). Patients were excluded according to the following criteria: (1) any known food intolerance, malabsorption, history of gastrointestinal surgery, or a history of organic gastrointestinal diseases; (2) antidepressant and prokinetic drugs intake for longer than 2 weeks before enrollment; (3) any current psychiatric disorders (e.g., psychosis, major depressive disorder, or drug and alcohol abuse as defined by diagnostic and statistic manual of mental health disorders, fourth edition text revision [DSM-IV-TR] criteria); (4) history of neurological disorders or head injury; (5) any contraindications to MRI scan. A complete medical history was taken for all patients, and a complete physical examination, blood test, and colonoscopy were performed in all patients.
Using block randomization design, patients were randomly divided into observation group (OG, n = 16) and control group (CG, n = 16). All patients in both groups were given spasmolytic agent (pinaverium bromide; 50 mg/day, tid), the OG group was added flupentixol-melitracen (10.5 mg/day, bid). There were no significant differences in age, gender, or IBS duration (P > 0.05) between the two groups (Table 1).
3.2. Data Acquisition
Evaluation of clinical symptoms at baseline and fMRI scans were completed before patients received medication. After 4 weeks of treatment, patients were reassessed and rescanned with the same process.
All patients were assessed by two trained physicians on their Hamilton depression scale (HAMD-17) (16, 17), Hamilton anxiety scale (HAMA-14) (18, 19), and gastrointestinal symptoms rating scale (GSRS) (20-22).
MRI images were acquired by using a whole-body 3.0 T scanner (GE Discovery MR-750, Waukesha, WI) with a 12-channel head coil. The collected images include the resting-state functional blood-oxygen-level-dependent (BOLD) images and high-resolution three-dimensional T1-weighted (3D-T1) image. Details of sequences and parameters used for image acquisition are shown in Table 2. Conventional MRI images were reviewed by two experienced radiologists, and no significant structural abnormalities were found in all subjects.
| Sequences | TR/TE, ms | FOV, mm | Slice thickness, mm | Matrix | FA, ° | |
|---|---|---|---|---|---|---|
| BOLD images | GRE-SS-EPI | 2000/30 | 192 × 192 | 4 | 64 × 64 | 90 |
| 3D-T1 image | SPGR | 8.16/3.18 | 256 × 256 | 1 | 256 × 256 | 8 |
Abbreviations: BOLD, blood-oxygen-level-dependent; FA, flip angle; FOV, field of view; GRE-SS-EPI, gradient-echo single-shot echo planar imaging; SPGR, spoiled gradient-recalled pulse; 3D-T1, three-dimensional T1-weighted; TR/TE, repetition time/echo time.
3.3. Data Analysis
The fMRI data analyzer was blinded to the treatment protocols and time session of each data. Resting-state fMRI data pre-processing was performed using the Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm/) and Data Processing Assistant for resting state-fMRI (data processing and analysis for resting state brain imaging [DPABI]) tool (23). Pre-processing: the image data of the first 10 time points of the resting-state scan are removed for slice-timing adjustment. Head-motion correction is eliminate the head motion in the X, Y, and Z axis of translation for more than 3 mm or rotation angle more than 3° data subjects; spatial standardization was carried out to warped the image into Montreal Neurological Institute (MNI) template (3 × 3 × 3 mm3). Subsequently, Linear drift of data was removed and 0.01 ∼ 0.08 Hz bandpass filter was used to eliminate physiological noise. By multiple linear regressions, several nuisance variables were removed from the data, including head-motion parameters, global brain signal, and average signal from white matter and ventricles.
After preprocessing, ReHo analysis was performed on the resting-state fMRI data. First, the Kendall’s coefficient on the time series of each voxel of the whole brain and its neighboring 26 voxels was calculated, which is the ReHo value of the voxels. Each voxel corresponded to a ReHo value, thus forming a whole-brain ReHo graph. The ReHo value of each voxel in the entire brain was divided by the mean value of all voxel ReHo values in the whole brain and standardized to obtain the average ReHo graph. Then spatial smoothing (full width at half maximum, FWHM = 6 mm) was performed before statistical analysis (13).
3.4. Statistical Analysis
Demographic and clinical data were analyzed using SPSS software (Version 18.0, SPSS Inc., Chicago, USA). Differences between group demographic data were analyzed using independent t-tests and χ2 tests. Differences in clinical data between groups were analyzed using independent t-tests at the pre-treatment and post-treatment. A 2 (therapy) × 2 (time) mixed model analysis of variance (ANOVA) was used to compare pre-treatment and post-treatment differences in the observation group and control group. Significance was set at P < 0.05. Statistical analyses of ReHo maps were performed using DPABI. To examine differences in ReHo values among patients with different medications, we performed a voxel wise mixed-model analysis of covariance (ANCOVA) with time (pre-treatment and post-treatment) and therapy (OG and CG) as factors. Post analyses were carried out using two-sample t-tests. Age, gender, framewise displacement (FD) and IBS duration were included as covariates. The statistical threshold was set at voxel level, P < 0.01; mass level, P < 0.05, with AlphaSim correction (24).
Finally, partial correlation analysis was performed to examine the association between changes in ReHo following treatment and reduced HAMD, HAMA and GSRS scores, controlling for age, sex, FD and IBS duration. The levels of significance were set at P < 0.05 without correction for multiple tests.
4. Results
4.1. Demographic Characteristics and Behavioral Results
Demographic details and clinical characteristics of all patients were summarized in Table 1 and Table 3. There were no statistically significant differences in GSRS, HAMD and HAMA scale scores between two groups before treatment (P > 0.05) (Table 3), indicating that the two groups had comparable levels of gastrointestinal symptoms, anxiety and depression before treatment. After 4 weeks of treatment, however, analysis of each subtest indicated that the observation group had significantly lower scores in GSRS (t = -4.122, P < 0.001), HAMD (t = -3.859, P = 0.001), and HAMA (t = -3.709, P = 0.001) compared to the control group. Two-factor mixed design ANOVA for GSRS (F = 5.819, P = 0.019), HAMD (F = 7.707, P = 0.007) and HAMA (F = 6.476, P = 0.014) scale scores also showed significant time and therapy interaction effects (Table 3). Compared with the baseline level, the scores of GSRS, HAMD and HAMA scales in the OG group were significantly reduced (P < 0.001), and GSRS score was significantly decreased in CG group after treatment (P < 0.05). HAMD and HAMA score did not show a statistically significant difference in the CG group before and after treatment (P > 0.05).
| Pre-treatment | Post-treatment | Time × group F value | |||||
|---|---|---|---|---|---|---|---|
| Observation group | Control group | Group difference t value | Observation group | Control group | Group difference t value | ||
| GSRS | 39.13 ± 12.06b | 37.25 ± 10.82c | 0.463 | 20.44 ± 4.15 | 29.75 ± 8.03 | -4.122d | 5.819b |
| HAMD | 10.31 ± 5.22b | 9.25 ± 6.21 | 0.524 | 2.25 ± 2.08 | 8.31 ± 5.93 | -3.859b | 7.707b |
| HAMA | 12.75 ± 5.21b | 11.88 ± 7.80 | 0.373 | 3.00 ± 2.19 | 9.63 ± 6.80 | -3.709b | 6.476c |
Abbreviations: GSRS, gastrointestinal Symptom Rating scale; HAMA, Hamilton anxiety scale; HAMD, Hamilton depression scale.
aValues are expressed as mean ± SD.
bP < 0.01.
cP < 0.05.
dP < 0.001.
4.2. Results of ReHo Comparisons in Mixed-Model ANCOVA and Post Analyses
4.2.1. Therapeutic Factor Was the Main Effect
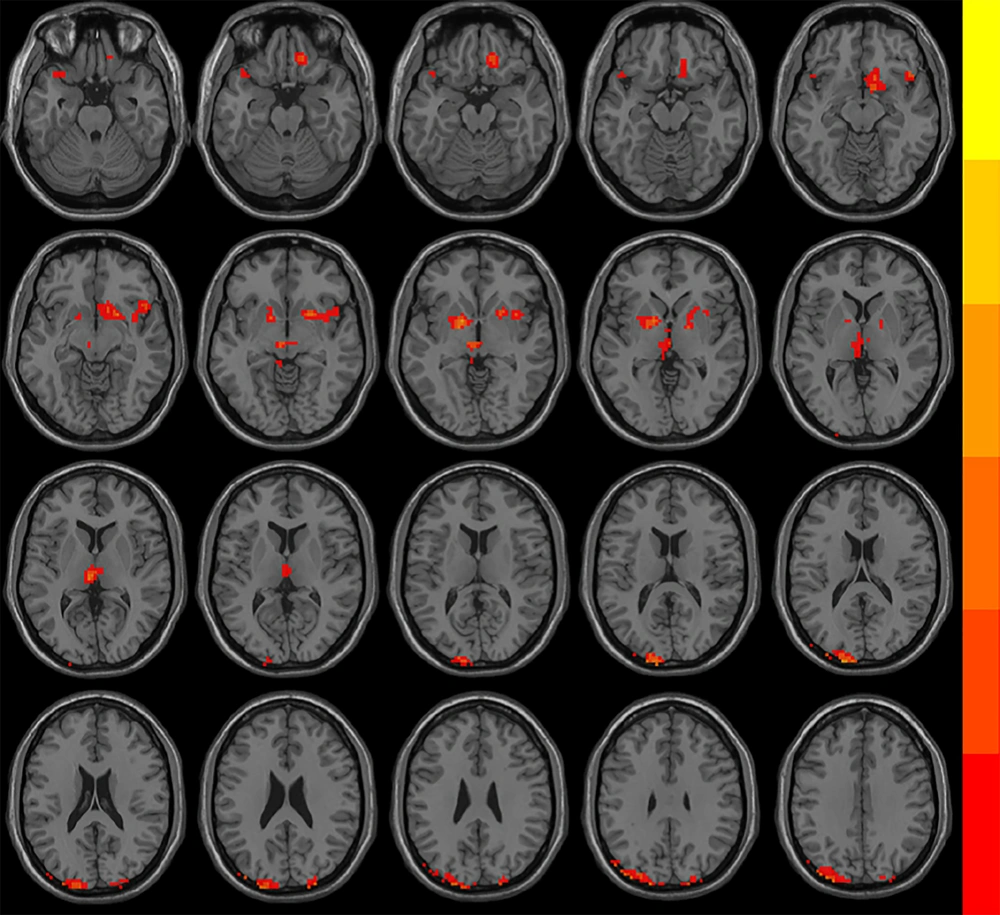
Following treatment, both groups exhibited significantly increased ReHo in the left superior and middle occipital gyrus, and the left parietal gyrus, whereas decreased ReHo was observed in the left thalamus and the lenticular nucleus. In addition, the OG group showed decreased ReHo in the left superior temporal gyrus, the right lenticular and putamen nucleus, the right inferior frontal gyrus, the right insula, the right gyrus rectus and the right subcallosal gyrus. The CG group showed increased ReHo in the right angular gyrus (AlphaSim corrected, with the level of voxle P < 0.01 and the level of mass P < 0.05) (Figure 1 and Table 4).
| Region | Peak MNI coordinates | Cluster size, mm3 | Peak intensity | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Both group pre-treatment > post-treatment | Thalamus (L) | -3 | -21 | 6 | 95 | 18.19 |
| Lenticular nucleus (L) | -15 | 3 | -3 | 64 | 19.72 | |
| Both group pre-treatment < post-treatment | Superior occipital gyrus (L) | -18 | -102 | 18 | 389 | 22.73 |
| Middle occipital gyrus (L)a | ||||||
| Superior parietal gyrus (L) | -45 | -69 | 51 | 91 | 20.99 | |
| Observation group pre-treatment > post-treatment | Superior temporal gyrus (L) | -36 | 21 | -27 | 59 | 14.19 |
| Lenticular-putamen nucleus (R) | 21 | 9 | -6 | 211 | 17.97 | |
| Inferior frontal gyrus (R)a | ||||||
| Insula (R)a | ||||||
| Gyrus rectus (R)a | ||||||
| Subcallosal gyrus (R)a | ||||||
| Control group pre-treatment < post-treatment | Angular gyrus (R) | 60 | -54 | 48 | 162 | 19.48 |
Abbreviations: L, left; MNI, Montreal Neurological Institute; ReHo, Regional homogeneity; R, right.
aSecondary peaks are in italic.
Brain regions with significantly different regional homogeneity (ReHo) values among groups when therapeutic factor was the main effect in the mixed-model analysis of covariance (ANCOVA) analysis (P < 0.05, Alphasim corrected). The left side of the images corresponds to the left side of the brain.
4.2.2. The Interaction Between Therapy and Time Was the Main Effect
The brain region with statistical difference was left precuneus (Figure 2A) (AlphaSim corrected, with the level of voxle P < 0.01 and the level of mass P < 0.05). Compared with baseline level, ReHo in the left precuneus in the OG group was increased, while that in CG group was decreased after treatment (Figure 2B). The changes of ReHo in the left precuneus were negatively correlated with reduction in the HAMD and HAMA scores in the OG group (r = -0.712, P = 0.009; r = -0.646, P = 0.023) (Figure 2C and Figure 2D).
Brain regions with significantly different ReHo when the interaction between treatment and group was the main effect in the mixed-model ANCOVA analysis. A, The region of different ReHo was the left precuneus (red); B, Followed treatment, ReHo in the left precuneus increased in the observation group (OG) and decreased in the control group (CG); C, D, In the OG group, there was a significant negative correlation between the changes of ReHo in the left precuneus and the reduction in the Hamilton depression rating scale (HAMD); and Hamilton anxiety rating scale (HAMA) scores.
5. Discussion
In the present study, we found that the combination of flupentixol and melitracen was more effective in improving anxiety, depression and gastrointestinal symptoms in IBS patients compared with basic treatment, further confirming that psychotropic drugs can not only control their own anxiety and depressive symptoms, but also contribute to significantly improving gastrointestinal symptoms in IBS patients. We found that both combined with anxiolytic/antidepressant group and only gastrointestinal spasmolytic drug group reduced resting-state brain activity in regions related to “pain matrix”, i.e., thalamus and lenticular nucleus, and increased in the posterior parieto-occipital cortex. In addition, the OG group resulted in more intensive and extensive decreases in brain activity in the cortices (prefrontal-temporal regions) and the limbic system, while the CG group resulted in greater increases in brain activity in the right angular gyrus. Significantly, we found that anxiolytic/antidepressant treatment was associated with increased ReHo in the left precuneus, and changes in this area were negatively correlated with the reduction in the HAMD and HAMA score.
The left precuneus is the “hub” of the default mode network (DMN) (25). DMN maintains the memory, attention and cognitive functions of the brain in resting state, which is an important node of functional connection of various brain regions (26). The precuneus and surrounding posteromedial areas are the highest metabolic brain regions in resting state, which maintain the self-consciousness function in the internal thinking process related to “self-reference” during rest state. The dysfunction of this region may lead to physiological lesions (25). Studies have reported that IBS patients showed high activation in the precuneus and surrounding areas under rectal stimulation, and decreased blood perfusion in this region and its functional connection with multiple brain regions under resting state, which were partially related to the depression score of patients. (27-30). Therapeutic studies have shown that abnormal activity and recovery of DMN, especially in the left precuneus, may be a state indicator for predicting anxiety and depression (31-33). When the interaction between therapy and time was the main effect, the region of different brain activity was the left precuneus. It should be noted that spontaneous activity in the precuneus was increased after combined treatment with flupentixol-melitracen, and the regional changes of ReHo values were negatively correlated with the changes of HAMA and HAMD scores, indicating that the increase of regional function correlated with the alleviation of mental symptoms. These results suggested that the precuneus would play a role in the pathophysiology for depression and anxiety; while, flupentixol-melitracen could modulate complex interactions of dopamine and serotonin (9, 10) that could also cause upregulation of spontaneous activity in the precuneus. Thus, we speculate that increased spontaneous activity in the precuneus may be a neuromechanism of selective serotonin reuptake inhibitors.
Followed combined anxiolytic/antidepressant treatment, IBS patients showed decreased ReHo in the left superior temporal gyrus, the right lenticular-putamen nucleus, the right inferior frontal gyrus, the right insula, the right gyrus rectus and the right subcallosal gyrus. A large number of fMRI studies have shown that abnormal prefrontal-limbic emotional circuits, including the prefrontal cortex, anterior cingulate cortex, hippocampus, basal ganglia, amygdala and insula, are potential neuropathological mechanisms for anxiety and depression (34). The major role of antidepressants is to reverse the negative bias in the information processing of the loop, so the neural activity of the loop can predict the efficacy of antidepressants (35-37). Some studies have found that the degree of atrophy in temporal lobe may be a predictor of the course of depression (38, 39), and the high activation of the superior temporal gyrus may be the neural basis for the deficits in cost-benefit decision-making in patients with depression disorder (40). In this study, changes in brain activity in the prefrontal-limbic system and temporal lobe were observed only in the OG group, suggesting that anxiolytic/antidepressant could significantly improve the mood disorders by regulating the emotional processing circuits, further promoting the recovery of gastrointestinal symptoms. The insula in the emotional circuit is not only related to mood change, but also closely related to the production and regulation of pain (41). We found that gastrointestinal symptoms in the CG group were partially relieved after basic treatment, but no significant changes in insular activity were observed. This result may be due to the symptoms of anxiety and depression that have not been significantly alleviated, leading to further amplification of central pain sensation.
When the therapeutic factor was the main effect, the values of ReHo in both groups after treatment were decreased in the left thalamus and the lenticular nucleus, and increased in the superior and middle occipital gyrus, and the left parietal gyrus compared to the unmedicated state. The thalamus is a key component of the steady-state afferent network and plays a crucial role in central processing of somatic and visceral pain. The most distinctive feature of the thalamus is its interconnection with the cerebral cortex, which can transmit pain information to the cerebral cortex (42). Task-state and resting-state fMRI study found that IBS patients had high thalamic activation and increased regional synchronization, which were positively correlated with the severity of gastrointestinal symptoms (43-47). The basal ganglia is involved in the integration of information with the cortex, thalamus and three specific pain processing regions (sensory, emotional/cognitive and endogenous/regulatory), which is an important part of the “pain matrix” (48, 49). Lenticular nucleus, an important part of the basal ganglia, serves a significant role in regulating pain (50). Chronic visceral pain can lead to a decrease in the volume of the left putamen (51). The occipital lobe has traditionally been associated with visual information processing. Although no visual impairment associated with chronic pain has been reported, some rodent studies have found that the occipital cortex reflects analgesic effects (52, 53). In addition, several human neuroimaging studies have reported changes in occipital lobe activity in patients with chronic pain (54-57). The parietal cortex integrates the signals transmitted by various sensory information and is related to sensory perception processing, self-consciousness and memory extraction. Stankewitz et al. (58) found persistent activation of the posterior parietal cortex (including the superior gyrus and subparietal lobules) in the perception of pain spatial location information. The results of our previous study also found ReHo changes in the parietal and occipital cortex in IBS patients (14). Based on the above studies, the findings of our study further indicate that abnormal neuronal activity in the pain network-related brain regions and the posterior parieto-occipital cortex in IBS patients could be recovered after improvement of gastrointestinal symptoms.
In conclusion, anxiolytic/antidepressant could effectively improve anxiety, depression and gastrointestinal symptoms in IBS patients, which may be related to the reversal of abnormal neural activity in the brain regions within the default network and the prefrontal-limbic-temporal emotional circuit in IBS patients. In addition, abnormal neuronal activity in the pain network-related brain regions and the posterior parieto-occipital cortex in IBS patients can be recovered after improvement of gastrointestinal symptoms.
This study also had limitations. Frist, the small sample size of this study may have affected the credibility and universality of the research results. Second, long-term follow-up studies are needed to determine whether further changes in clinical symptoms and brain activity occur at later stages. Third, ReHo focuses on the functional areas of the entire brain, but it may be ignoring the functional characteristics of specific brain regions. Last but not the least, the results of this study may be influenced by non-therapeutic factors (for example, the treatment behavior itself leads to differences in the scale and fMRI results). In the future, it is necessary to expand the sample size, improve the consistency of the sample size, add the placebo group, and conduct multimodal research to verify each other.