1. Background
Aortic diseases are silent killers associated with a high prevalence of cardiovascular mortality (1). These diseases are common and lack specific treatments to prevent their progression; hence, patients must be monitored until they require interventional procedures (1-3). Aortic aneurysms are pathological dilatations of an area in the vessel with the potential for rupture and progression to other parts. The threshold for this definition is an aortic diameter of 40 mm in the thoracic aorta and 30 mm in the abdominal aorta (4). The abdominal aortic aneurysm (AAA) is the most common form of aortic aneurysm, with the infrarenal part being the most frequently involved area of the vessel. In the thoracic aortic aneurysm, the most commonly involved area is the ascending part. Risk factors for aortic aneurysms include positive family history, aging, hypertension, hyperlipidemia, smoking, coronary artery disease, chronic obstructive pulmonary disease, and infections. Untreated cases can be complicated by acute aortic syndromes such as aortic dissection, which can be fatal in many cases (4). Risk factor management in AAA plays a significant role in decelerating disease progression.
There are many collaborative risk factors. Previous studies have shown that most patients have concurrent atherosclerosis; a causal relationship has yet to be established (5). Obesity is an independent risk factor for atherosclerosis and coronary artery disease (6, 7) and is considered an AAA risk factor by several investigators (8). Ectopic adipose tissue is a source of fat in non-classic areas (9) and seems to have a systemic effect on cardiovascular events by causing hypoxia, inflammation, and oxidative stress (10-13). Perivascular adipose tissue is ectopic fat with possible local effects on the vessels (14-16). It can also secrete many bioactive substances, including endothelial growth factor, tumor necrosis factor α, leptin, adiponectin, insulin-like growth factor, and interleukin-6. These substances have the potential to increase blood pressure and produce aneurysms (1-3). Another mechanism of AAA formation is macrophage infiltration and cytokine release in abdominal periaortic tissue, increasing angiotensin-II secretion (17). Further, central obesity is an excess accumulation of fat in the abdominal area, particularly due to excess visceral fat, and is associated with local aortic and peripheral arterial diseases (18).
Periaortic adipose tissue can be measured by multidetector cardiac computed tomography (MDCT) scanning (19, 20). Although studies in this area are limited, using MDCT to evaluate periaortic adipose tissue can significantly contribute to identifying high-risk patients for a preventive plan (19).
2. Objectives
The present study aimed to measure periaortic adipose tissue and investigate its association with the indexed aortic diameter according to MDCT findings. Additionally, the prevalence of aortic aneurysms and other risk factors, including hyperlipidemia and diabetes mellitus, in the study group was evaluated.
3. Methods
The current retrospective and cross-sectional study, conducted in 2019, enrolled 149 patients who underwent thoracic and abdominal CT angiography at a tertiary center for cardiovascular diseases. The participants were aged 20 years and above and had an indication for CT angiography. Exclusion criteria included the presence of rheumatic heart diseases, congenital heart diseases, connective tissue diseases, bicuspid aortic valves, aortic stenosis, aortic insufficiency, uncontrolled hypertension, and any history of cardiovascular surgeries.
According to a previous study on the relationship between TAT, VAT, and BMI indicators, the correlation of these indicators with the diameter of the thoracic aorta was reported as 0.26, 0.28, and 0.34, respectively. Using these results and the sample size formula for correlation studies, and including a type I error of 0.05 and power of 90%, the required sample sizes were 150, 130, and 87, respectively. Therefore, the required sample size in this study was considered to be 151 patients who were candidates for aortic CT angiography.
The study process was fully explained to the patients, who were recruited without any extra payment. The patients' data were recorded and saved confidentially. The study protocol was approved by the Ethics Committee of the Iran University of Medical Sciences (IUMS) (code: IR.IUMS.FMD.REC.1399.073). In the first stage, demographic data, including age, sex, Body Mass Index (BMI), and history of hypertension or diabetes mellitus, were collected. The patients underwent CT angiography as part of their clinical care after the initial diagnosis.
In the CT scan process, 70 mL of Omnipaque 350 (contrast agent) was administered intravenously using an automatic injection driver system. Adipose tissue volume was measured using an HU range of -45 to -190, applied to identify pixels containing adipose tissue. In a study by Schlett et al. (19), adipose volume was measured via a semiautomatic segmentation technique requiring manual definition of the tissue border. Images were taken from the surface of the carina with a thickness of 2.5 mm for the thoracic aorta and the upper surface of the S1 vertebra for the abdominal aorta. In all cases, the ascending aortic diameter was measured at the level of the right pulmonary artery. Additionally, the diameter of the abdominal aorta was measured at a distance of 5 cm from the level of the aortic bifurcation (middle part of the abdominal aorta) and from the outer edge to the outer aortic wall by an experienced radiologist. The procedure resulted in a 6.75 cm column of fat: 27 slices surrounding the thoracic aorta and a 5 cm column of adipose tissue in the abdominal aorta. The fat area was defined by pixels with a characteristic HU (i.e., -45 to -190 [window center: -120 HU]).
Thoracic aortic aneurysms were defined as those greater than 4 cm or 40 mm in diameter, and AAAs were defined as those exceeding 3 cm or 30 mm in diameter. Body Mass Index was calculated as body weight in kilograms divided by height in meters squared. The aortic size index was calculated by dividing the aortic size by BMI. Additionally, the diameters of the aorta and adipose tissue were measured.
3.1. Statistical Analysis
Continuous variables were expressed as the mean ± standard deviation (SD) and categorical variables as numbers (%). Comparisons were performed using the chi-square or Fisher exact tests for categorical variables, the independent t-test for normally distributed variables, and the Mann–Whitney test for non-normally distributed variables. A P-value of less than 0.05 was considered statistically significant. The data were analyzed using SPSS software, version 16.
4. Results
The study population consisted of 149 patients, including 47 women (31.5%) and 102 men (68.5%), with an age range of 20 to 80 years (mean = 60.55 years). The BMI range was from 17 to 39.3 (mean = 25.65). The prevalence rates of thoracic aortic aneurysm and AAA were 8.7% (n = 13) and 24.8% (n = 37), respectively. The average diameters of the thoracic and abdominal aortae were 29.9 mm and 18.43 mm, respectively. Additionally, the average perivascular fat volume was 13.99 mL in the thoracic aorta and 16.99 mL in the abdominal aorta.
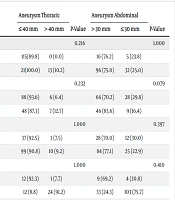
No significant differences existed in terms of sex, mean age, and BMI between the two groups with and without thoracic aortic aneurysms (P = 1.00, P = 0.491, and P = 0.376, respectively). However, the prevalence of AAA in men was significantly higher than in women (P = 0.025). The mean age in the group of patients with AAAs and abdominal aortic diameters exceeding 30 mm was significantly higher (P = 0.003). There was no significant difference in BMI between patients with and without AAAs (P = 0.483), similar to those with and without thoracic aortic aneurysms (Table 1).
| Clinical Variables | Aneurysm Thoracic | P-Value | Aneurysm Abdominal | P-Value | ||
|---|---|---|---|---|---|---|
| ≤ 40 mm | > 40 mm | > 30 mm | ≤ 30 mm | |||
| Diabetes mellitus | 0.216 | 1.000 | ||||
| Yes | 115(89.8) | 0 (0.0) | 16 (76.2) | 5 (23.8) | ||
| No | 21(100.0) | 13 (10.2) | 96 (75.0) | 32 (25.0) | ||
| Hypertension | 0.232 | 0.079 | ||||
| Yes | 88 (93.6) | 6 (6.4) | 66 (70.2) | 28 (29.8) | ||
| No | 48 (87.3) | 7 (12.7) | 46 (83.6) | 9 (16.4) | ||
| Dyslipidemia | 1.000 | 0.397 | ||||
| Yes | 37 (92.5) | 3 (7.5) | 28 (70.0) | 12 (30.0) | ||
| No | 99 (90.8) | 10 (9.2) | 84 (77.1) | 25 (22.9) | ||
| Family history of cardiovascular diseases | 1.000 | 0.410 | ||||
| Yes | 12 (92.3) | 1 (7.7) | 9 (69.2) | 4 (30.8) | ||
| No | 12 (8.8) | 24 (91.2) | 33 (24.3) | 103 (75.7) | ||
| Coronary artery disease | 0.216 | 0.670 | ||||
| Yes | 2 (4.7) | 41 (95.3) | 12 (27.9) | 31 (72.1) | ||
| No | 11 (10.4) | 95 (89.6) | 81 (76.4) | 25 (23.6) | ||
| Cerebrovascular accident | 0.371 | 0.014 a | ||||
| Yes | 1 (20.0) | 4 (80.0) | 1 (20.0) | 4 (80.0) | ||
| No | 12 (8.3) | 132 (91.7) | 111 (77.1) | 33 (22.9) | ||
| Smoking | 0.014 a | 0.032 a | ||||
| Yes | 45 (83.3) | 9 (16.7) | 19 (35.2) | 35(64.8) | ||
| No | 4 (4.2) | 91(95.8) | 18 (18.9) | 77(81.1) | ||
Clinical Characteristics of the Study Population
A significant correlation was found between the fat volume around the thoracic aorta and the fat volume around the abdominal aorta (P < 0.001, r = 0.504). There was also a significant relationship between age and the fat volume around the thoracic and abdominal aortae (P < 0.001 and P < 0.007, r = 0.379 and r = 0.222, respectively). Additionally, there was a significant relationship between BMI and the fat volume around the abdominal aorta (P = 0.044, r = 0.165), whereas the relationship between BMI and the volume of fat around the thoracic aorta was not significant (P = 0.795, r = 0.021).
Perivascular adipose tissue in the thoracic and abdominal parts of the aorta was evaluated according to several factors including diabetes mellitus (DM), hypertension, dyslipidemia, smoking, positive family history of cardiovascular disease, history of cerebrovascular accidents, and coronary artery disease. Significant correlations were found between hypertension and smoking with perivascular adipose tissue in the aortic aneurysm in both thoracic and abdominal parts. A significant correlation was also found between coronary artery disease and thoracic aortic aneurysm (P < 0.05) (Table 2).
| Clinical Characteristics (Middle) | Perivascular Adipose Tissue (Thoracic)Median (IQR) | P-Value | Perivascular Adipose Tissue (Abdominal)Median (IQR) | P-Value |
|---|---|---|---|---|
| Diabetes mellitus | 0.313 | 0.470 | ||
| Yes | 11.08 (7.97 - 20.82) | 11.00 (8.90 - 19.62) | ||
| No | 10.70 (7.08 - 17.00) | 10.90 (7.80 - 19.62) | ||
| Hypertension | 0.004 | 0.001 | ||
| Yes | 12.00 (8.82 - 18.13) | 12.35 (8.97 - 24.30) | ||
| No | 8.60 (5.96 - 15.80) | 8.90 (6.80 - 13.00) | ||
| Dyslipidemia | 0.719 | 0.581 | ||
| Yes | 10.00 (7.83 - 17.98) | 11.00 (7.93 - 21.30) | ||
| No | 11.00 (6.85 - 17.55) | 11.00 (7.78 - 18.15) | ||
| Family history of cardiovascular diseases | 0.527 | 0.561 | ||
| Yes | 10.00 (5.65 - 17.46) | 10.50 (6.50 - 26.35) | ||
| No | 10.97 (7.53 - 17.88) | 11.00 (7.93 - 19.02) | ||
| Cerebrovascular accident | 0.079 | 0.193 | ||
| Yes | 14.13 (13.73 - 21.70) | 14.67 (9.64 - 58.45) | ||
| No | 10.46 (7.08 - 17.68) | 10.72 (7.80 - 19.02) | ||
| Coronary artery disease | 0.031 | 0.187 | ||
| Yes | 12.00 (9.40 - 17.90) | 11.26 (8.99 - 21.70) | ||
| No | 10.00 (6.38 - 17.48) | 10.75 (7.11 - 17.64) | ||
| Smoking | 0.000 | 0.001 | ||
| Yes | 16.28 (10.75 - 24.30) | 13.32 (10.00 - 26.10) | ||
| No | 8.90 (6.00 - 14.00) | 9.90 (6.90 - 13.60) |
Comparison of Central Indices and Fat Distribution Around the Aorta in Terms of Demographic and Clinical Variables
A significant relationship existed between the fat volume around the thoracic aorta and both the diameter of the thoracic aortic aneurysm and the diameter of the AAA (P < 0.001), which was the most significant result of the present study. Moreover, a clear correlation was detected between the fat volume around the abdominal aorta and the diameter of the abdominal aorta (P < 0.031). The effect of BMI was eliminated via another examination using the size of the indexed aorta. Once again, a significant relationship was found between the fat volume around the thoracic aorta and the indexed thoracic aorta (P < 0.001). Additionally, no meaningful relationship existed between the volume of fat around the abdominal aorta and the size of the indexed thoracic aorta.
5. Discussion
In the present cross-sectional study, we sought to determine the association between the aortic diameter and the periaortic adipose tissue volume. In this investigation, we assessed the correlation between the volume of periaortic fat tissue and the aortic aneurysm and found a meaningful relationship not only in the thoracic aortic aneurysm but also in the AAA.
In the Framingham study (as cited in Schlett) (19, 21), conducted in the United States, periaortic adipose tissue was evaluated using CT scanning. The results showed a significant correlation between the quantitative volume of adipose tissue and the aortic diameter in both thoracic aneurysms and AAAs, with the correlation being more significant in the thoracic aorta. Dias-Neto et al. (22) reported that the volume of perivascular adipose tissue was significantly higher in patients with AAAs than in subjects without aneurysms.
In their investigation, Cronin et al. (23) showed that the ratio of the visceral fat tissue volume to the total fat tissue volume of the body had no significant correlation with the size of the AAA, which does not align with our results.
Our findings demonstrated a significant relationship between the volumes of periaortic thoracic and abdominal adipose tissue (P < 0.001). This correlation was confirmed in both thoracic and abdominal aneurysms even after we indexed the aortic size with BMI. The volume of the periaortic AAA was correlated with the indexed values, unlike the thoracic aortic aneurysm.
The literature features several studies on aortic aneurysms and related factors. Kaya et al. (24) studied 432 patients over a follow-up period of three years to evaluate the relationship between periaortic fat tissue measured by CT scanning and the prevalence of long-term cardiovascular events. At the end of the follow-up period, cardiovascular events were reported in 44 patients, in whom the volume of periaortic adipose tissue was significantly higher than that in the other patients (P = 0.001).
Başpınar et al. (25) used CT scanning to measure epicardial fat tissue in patients with rheumatic arthritis and found a significant correlation between this tissue and the diameter of the aortic and pulmonary arteries.
Toufan et al. (26) utilized echocardiography to assess the correlation between periaortic fat tissue volume and aortic diameter, reporting a significant relationship between the two variables in different areas of the aorta. They also showed an independent correlation between aortic diameter and factors such as dyslipidemia, aging (> 65 years), and BMI.
5.1. Limitation
The primary limitation of the present study was its small sample size due to the wide inclusion and exclusion criteria devised. We utilized the past medical records of our patients and collected only limited data for analysis purposes. Additionally, the measurement of periaortic adipose tissue is a novel and challenging procedure.
Further studies with larger statistical populations and follow-ups could confirm the results of our investigation and clarify the long-term efficacy of the treatment.
5.2. Conclusions
Preventing aortic aneurysms requires controlling risk factors such as smoking and obesity. It is also crucial to follow up with patients who have aortic aneurysms. According to our study and several other investigations on different ethnicities and races, periaortic adipose tissue has a significant correlation with aortic aneurysms. In candidates for aortic CT angiography, we recommend the measurement of periaortic adipose tissue as additional data for better management.
.jpg)