1. Background
Coronary artery disease (CAD) is a leading cause of death. Recent studies have demonstrated an upward trend in its prevalence due to sedentary lifestyles, poor diets, and a consequent lack of control over related risk factors (1). Applying different therapeutic approaches, including lifestyle modification, various medications, and revascularization in severe cases, can improve disease-related outcomes, long-term patient survival, and various components of quality of life (2, 3). Regarding revascularization, coronary artery bypass grafting (CABG) surgery and percutaneous coronary intervention (PCI) have significantly reduced mortality and morbidity in patients by improving blood flow to the myocardial tissue, balancing tissue supply and demand, and ultimately enhancing ventricular systolic and diastolic function (4, 5).
Traditionally, dynamic indicators such as left ventricular ejection fraction (LVEF) or the diameters of the heart muscles, cavities, and vessels have been used to assess ventricular function. However, the emphasis has recently shifted toward more accurate parameters, such as longitudinal myocardial strain, in evaluating changes in myocardial function following revascularization. Speckle tracking echocardiography (STE), a new diagnostic modality, provides an opportunity to assess deformation in the myocardium's circumferential, longitudinal, and radial planes, correlating well with changes in mechanical function (6). Speckle motion analysis can assess the velocity and strain of myocardial tissue independently of cardiac translation (7). The quantitative evaluation of ventricular functional state by STE offers a significant advantage.
Several studies on the echocardiographic assessment of RV function after cardiac surgery have evaluated these parameters. Tricuspid annular plane systolic excursion (TAPSE) and s’ appear to be reduced in subjects undergoing surgery for both congenital and acquired heart diseases (8). However, these changes may not necessarily indicate RV dysfunction (9). Here, we aimed to investigate various echocardiographic RV function indices to determine which are most useful for evaluating RV function after CABG.
2. Objectives
The present study assessed right ventricular functional changes using STE following CABG.
3. Methods
3.1. Study Methodology
This prospective before-and-after study was conducted on patients with a definite diagnosis of CAD (confirmed by coronary angiography) who were candidates for on-pump CABG at Faghihi and Namazi hospitals in Shiraz, Iran, in 2019 and 2020.
3.2. Target Population
The study population included all CABG candidates aged 30 to 70 years without a previous history of angioplasty or other coronary interventions and without a history of acute coronary syndrome or myocardial pacing. All patients had severe coronary artery stenosis (> 70% stenosis) on angiography. We excluded subjects with any evidence of congenital heart diseases, chronic obstructive lung disease, valvular heart diseases (in initial echocardiography assessment), or reduced LVEF (less than 35%). Additionally, patients with a poor echocardiographic window were excluded. A total of 60 patients completed the study.
3.3. Sampling and Methods
All cases underwent a detailed medical history assessment. Before scheduling CABG, all patients were evaluated using speckle-tracking echocardiography (STE) (General Electric Vivid E9, Norway) and subsequently underwent CABG using the same technique. All patients received standard post-operative care and medications in line with current European guidelines (reference). Six weeks after CABG, echocardiography was performed again.
All echocardiographic evaluations were conducted using General Electric's (GE) E9 machine with an M5ScD probe and 1.5 - 4.6 MHz bandwidth. Each patient underwent two rounds of echocardiography performed by two expert operators who were blinded to the treatment to reduce bias and inter-observer variability. The mean of these examinations is reported. Echocardiography was performed in the left lateral decubitus position, and the following parameters were assessed by STE before and after surgery: Fractional area change (FAC), left ventricular end-diastolic diameter (LVEDD), right ventricular end-diastolic diameter (RVEDD), tricuspid annular plane systolic excursion (TAPSE), S’ tissue Doppler imaging (S’TDI), global 2D longitudinal right ventricular strain, and right ventricle free wall 2D longitudinal strain. The primary endpoint was to assess the changes in these parameters following CABG.
3.4. Statistical Analysis
Results are presented as mean ± standard deviation (SD) for quantitative variables and as frequency (percentage) for categorical variables. The significance of changes in study parameters was assessed using the paired t-test or the non-parametric Wilcoxon test. Two-sided P-values ≤ 0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 16.0 for Windows (IBM, Armonk, New York).
4. Results
This study involved 60 CABG candidates with a mean age of 66 ± 9 years. Most patients (71.7%) had a prior history of myocardial infarction. Nearly two-thirds (63.3%) of patients had three-vessel coronary artery disease. The most prominent risk factor was hypertension (88.3%). The mean number of grafts was 3.0 ± 0.8, with a bypass time of 123 ± 39 minutes. Table 1 presents the baseline and operative characteristics of the patients.
| Variables | Values |
|---|---|
| Age, y | 66 ± 9 |
| Male sex | 48 (80) |
| Prior myocardial infarction | 43 (71.7) |
| Hypertension | 53 (88.3) |
| Diabetes mellitus | 25 (41.6) |
| Atrial fibrillation | 7 (11.7) |
| Current smoker | 14 (23.3) |
| Body mass index, kg/m2 | 30.2 ± 5.8 |
| COPD | 20 (33.3) |
| Cerebrovascular accident | 17 (28.3) |
| Peripheral artery disease | 27 (46.7) |
| Prior cardiac surgery | 3 (5.0) |
| Laboratory values | |
| GFR, mL/min/1.73 m2 | 85 ± 36 |
| Hemoglobin, g/dL | 13.4 ± 1.8 |
| Medications | |
| Beta blocker | 55 (91.7) |
| ACEI/ARB | 43 (71.7) |
| Spironolactone | 4 (6.7) |
| Digoxin | 5 (8.3) |
| Diuretics | 26 (43.3) |
| Operative parameters | |
| Number of grafts | 3.0 ± 0.8 |
| Bypass time, min | 123 ± 39 |
| Ischemic time, min | 80 ± 27 |
| Number of diseased coronary vessels | |
| 1 vessel | 3 (5.0) |
| 2 vessels | 19 (31.7) |
| 3 vessels | 38 (63.3) |
| Left main disease | 20 (33.3) |
Abbreviations: COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate.
a Values are expressed as No. (%) or Mean ± SD.
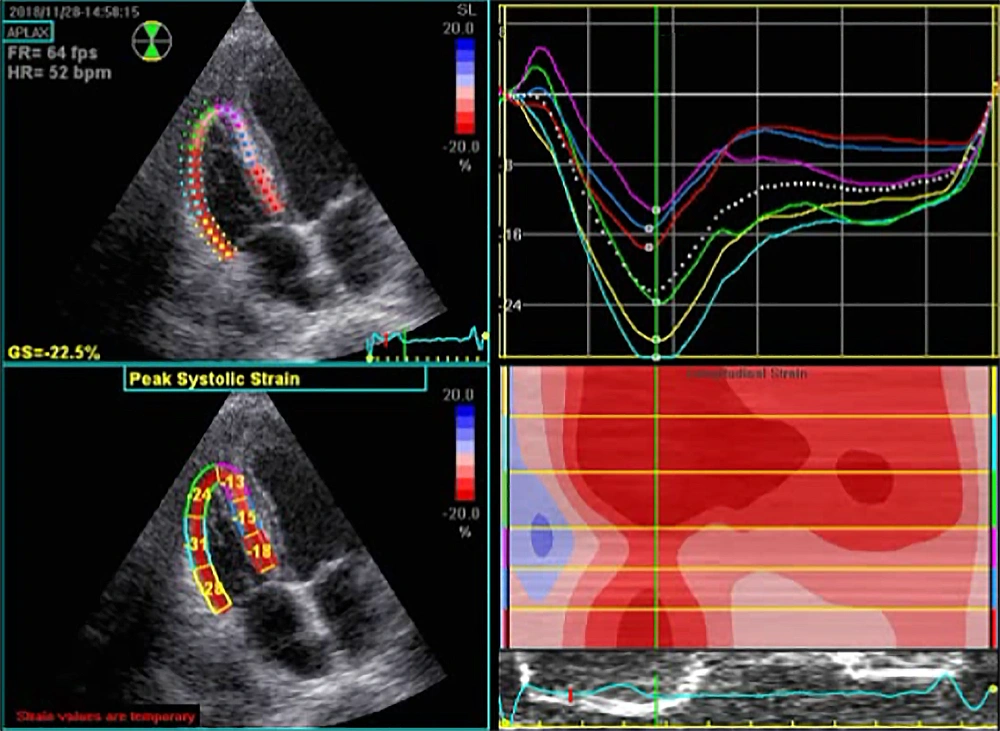
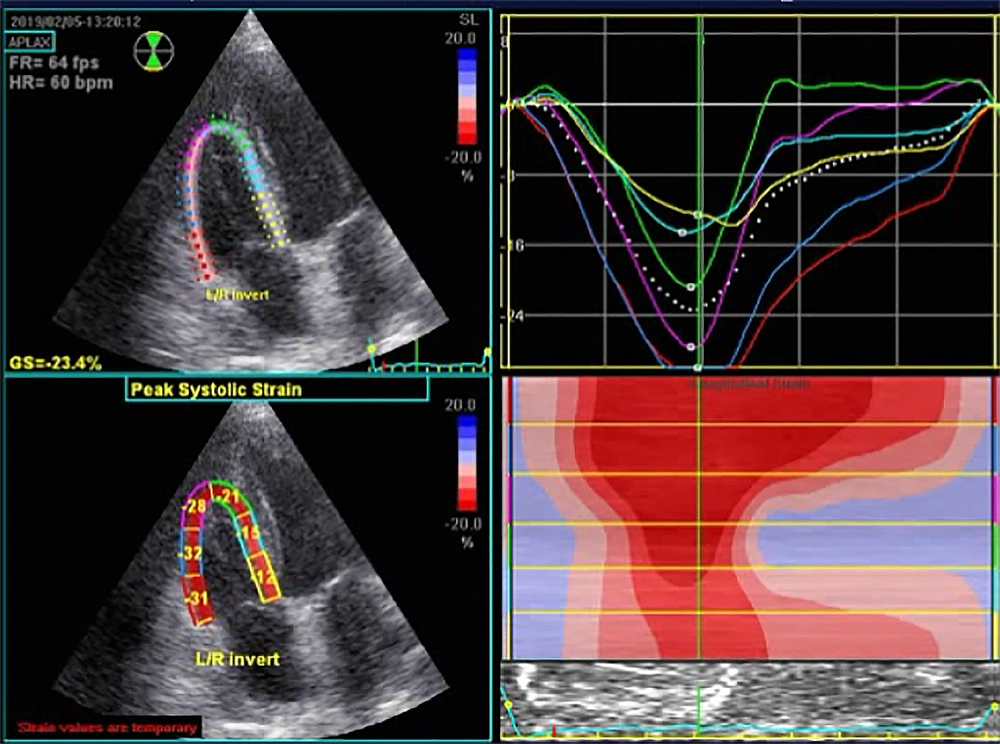
Echocardiographic parameters before and after CABG are summarized in Table 2. Among all myocardial parameters assessed by STE before and six weeks after CABG, significant changes were found in the two parameters of TAPSE and S’ tissue Doppler imaging. However, other parameters such as left ventricular end-systolic diameter, right atrial area, right ventricular end-diastolic area, right ventricular end-systolic area, right ventricular fractional area change, right ventricular end-diastolic diameter, right ventricular global 2D longitudinal strain (Figures 1 and 2), and right ventricular free wall longitudinal strain did not change significantly. Nonetheless, significant improvements in left ventricular end-diastolic diameter (from 47.11 ± 4.60 mm to 47.80 ± 4.26 mm, P = 0.01) and ejection fraction (from 55.00 ± 3.83% to 56.14 ± 4.03%, P = 0.03) were recorded. None of the patients had severe valve diseases requiring intervention.
| Parameter | Before | After | P-Value |
|---|---|---|---|
| Left ventricular end-diastolic diameter | 47.11 ± 4.60 | 47.80 ± 4.26 | 0.01 |
| Left ventricular end-systolic diameter | 30.42 ± 4.57 | 30.91 ± 3.97 | 0.13 |
| Left ventricular ejection fraction | 56.14 ± 4.03 | 55.00 ± 3.83 | 0.03 |
| Right atrial area | 12.31 ± 2.31 | 12.56 ± 2.24 | 0.16 |
| Right ventricular end-diastolic area | 13.88 ± 3.67 | 13.96 ± 3.92 | 0.77 |
| Right ventricular end-systolic area | 6.53 ± 2.30 | 6.51 ± 2.46 | 0.84 |
| Right ventricular fractional area change | 53.08 ± 6.80 | 53.47 ± 6.32 | 0.42 |
| Right ventricular end-diastolic diameter | 26.34 ± 3.10 | 26.62 ± 2.93 | 0.77 |
| Right ventricular global 2D longitudinal strain | -18.83 ± 2.88 | -18.79 ± 2.66 | 0.88 |
| Right ventricular free wall longitudinal strain | -21.29 ± 3.36 | -20.86 ± 3.17 | 0.12 |
| Tricuspid annular plane systolic excursion | 20.82 ± 2.53 | 14.60 ± 1.84 | 0.01 |
| S’ tissue Doppler imaging | 11.79 ± 1.99 | 8.80 ± 0.88 | 0.01 |
5. Discussion
The present study assessed right ventricular functional changes using STE following CABG. The 2010 ASE guidelines recommend standard echocardiography to evaluate the right heart in adults by assessing parameters such as TAPSE, S’ tissue Doppler imaging index, right ventricular FAC, and the myocardial performance index. Measuring multiple indicators provides a more reliable estimate of right ventricular function (10). In this study, three of these indicators were examined, showing significant changes in TAPSE and S’ tissue Doppler imaging indices. However, the change in FAC did not reach statistical significance. This index represents the percentage of surface change within the right ventricle between diastole and systole, providing an estimate of the overall right ventricular systolic function. The FAC marker helps independently indicate heart failure and can predict sudden death from myocardial infarction, stroke, and mortality following pulmonary embolism (11). However, our study indicates that it remains unchanged following CABG, at least in the first weeks after treatment.
Myocardial deformity assessed according to strain is more valuable than the study of wall motion (velocity and displacement) in diagnosing regional cardiac abnormalities (12). Several recent studies have shown that two-dimensional signaling (2D-STI) imaging is a practical method for assessing right ventricular function. Longitudinal strain is independent of the overall movement of the heart, thus allowing the analysis of regional deformation of the heart in different parts of the right ventricle (13-15). However, our study showed no significant changes in the two related parameters, namely global 2D longitudinal right ventricular strain and right ventricular free longitudinal strain, after CABG.
In a study by Abdelmoneum et al. in 2019 (16), a significant decrease in peak right ventricle systolic velocity and TAPSE and an increase in right ventricle fractional area change were reported. In their study, and contrary to our observation, a significant decrease in right ventricle global longitudinal strain and right ventricle free wall longitudinal strain was also detected. However, similar to our study and Korshin et al. (17), although TAPSE and RV longitudinal displacement were significantly reduced after CABG, RV speckle tracking strain did not change significantly. Khani et al. (18) recorded a statistically significant decrease in RVGLS from (-19 to -11) one week after CABG. Factors such as operator experience in assessing myocardial dynamic parameters, patient evaluation time, and cardiovascular comorbidities may explain this discrepancy in the findings of various studies. Hence, it remains unclear whether surgery can affect the right ventricular myocardial infarction indices, highlighting the need for further studies.
As a secondary outcome, we found the change in LVEDD following treatment to be 0.684 mm, which was statistically significant although not clinically significant. While the left ventricular diameter at the end of systole increased after treatment, this did not reach statistical significance. Left ventricular diameters are among the main indices for assessing ventricular function and can be easily measured by echocardiography during LVEF measurement. Previous studies have highlighted the importance of LV size in relation to cardiac mortality. A study of patients with LV disorders showed that increased LV diastolic diameter was associated with increased cardiovascular mortality (19). Similarly, another study showed that a reduced risk of death or heart failure was directly related to decreasing left ventricular diastolic diameter (20). Some studies in specific populations suggest that LV diameter can be useful in assessing the risk of unpredictable sudden cardiac death (21). Hence, while our study primarily focused on RV parameters, the value of indices related to LV function is undeniable.
The limitations of this study included its single-center nature and lack of long-term follow-up. Furthermore, we did not investigate pathophysiological mechanisms that could alter standard echocardiographic parameters after CABG. Additionally, we omitted patients with a limited echocardiographic window as strain imaging and speckle tracking depend on an optimal acoustic window, which is not always available. In contrast, TAPSE and S' are less dependent on the acoustic window.
5.1. Conclusions
This study assessed right ventricular functional changes using STE over six weeks following CABG. Our findings indicate that CABG induces changes in TAPSE and S’ tissue Doppler imaging indices but does not affect right ventricular global 2D longitudinal strain or free wall longitudinal strain.