1. Background
Coronavirus SARS-CoV-2, known to cause COVID-19, primarily results in a respiratory infection but also has associated cardiovascular effects, regardless of pre-existing cardiovascular disease (1, 2). It has been shown that the prevalence of cardiac injury, one of the most common complications in COVID-19 patients, is estimated to be 19.7% (1). A statistically significant relationship has been observed between cardiac injury in these patients and hospital mortality (2).
Considering the clinical prognostic characteristics of COVID-19, it is evident that cardiovascular complications play a significant role in the mortality and prognosis of these patients. Arrhythmias and other cardiovascular symptoms have frequently been reported in COVID-19 patients (3, 4). These symptoms may be related to myocarditis associated with infection, ischemia, and systemic inflammatory stimulation, often referred to as a cytokine storm (5). Recently, it has been suggested that arrhythmias could be due to hypoxia, caused by direct viral involvement of lung tissue, or myocardial ischemia (2). Myocardial strain caused by pulmonary hypertension or myocarditis, abnormal host immune responses, electrolyte imbalances, intravascular volume disturbances, and medication side effects could also contribute to arrhythmias (3, 6). Furthermore, arrhythmias have been more prevalent in patients hospitalized in the intensive care unit (ICU) (6-8). In a case study of 121 patients with SARS-CoV-2, 14.9% experienced bradycardia as a result of a temporary event (1). Banach examined two cases of pathological sinus node dysfunction (SND) related to COVID-19 and stated that patients with COVID-19 need careful monitoring for the progression of bradyarrhythmia and hemodynamic instability (3). Additionally, Pesce et al. explained that despite managing bradycardia with a permanent or temporary pacemaker, there was a high rate of short-term morbidity and mortality due to COVID-19 complications (2). This suggests that the relationship between newly developed bradyarrhythmia and significant outcomes may influence the management strategies for patients with severe COVID-19.
2. Objectives
Cardiovascular complications of COVID-19 infection, such as myocardial injury and bradyarrhythmia, can increase mortality and significantly affect the prognosis of these patients. Given the severity and frequency of these complications, there is a critical need for further research to develop reliable prognostic tools based on clinical characteristics. These tools would enhance care and treatment strategies for patients suffering from myocardial injury due to COVID-19. The aim of this study was to identify prognostic indicators based on the clinical characteristics of patients with COVID-19 who experienced myocardial injury. This study also seeks to provide a detailed analysis of the evidence associated with these prognostic indicators and their implications for patient outcomes.
3. Methods
3.1. Participants and Setting
In our study, we analyzed 50 patients with confirmed COVID-19 who exhibited significant, sustained bradycardia during their stay at Al-Zahra Hospital from July 22, 2020, to January 19, 2021. All admitted patients presented with symptoms such as respiratory issues (e.g., cough, dyspnea), myalgias, headaches, gastroenteritis (e.g., nausea, vomiting, diarrhea), disturbances in smell or taste, and an oxygen saturation level below 92% in room air (Table 1).
| Parameters | No. (%) |
|---|---|
| Underlying disease | |
| Hypertension | 24 (48) |
| Diabetes mellitus | 19 (38) |
| Ischemic heart disease | 12 (24) |
| Hypothyroidism | 6 (12) |
| Previous cerebrovascular accident | 5 (10) |
| Coronary artery bypass graft surgery | 2 (4) |
| Chronic kidney disease | 3 (6) |
| End-stage renal disease | 2 (4) |
| Heart failure | 3 (6) |
| Bronchiectasis | 1 (2) |
| Intracerebral hemorrhage | 1 (2) |
| Previous AF | 2 (4) |
| Parkinson | 1 (2) |
| Pregnancy | 1 (2) |
| HRCT results | |
| Mild | 8 (16) |
| Moderate | 17 (34) |
| Moderate to severe | 12 (24) |
| Severe | 8 (16) |
| Atypical | 5 (10) |
The Frequency of Underlying Diseases and HRCT Results
3.2. Inclusion and Exclusion Criteria
- Inclusion criteria: (1) Patients with confirmed COVID-19 infection; (2) patients with no prior history of bradycardia; (3) patients who exhibited significant, sustained bradycardia (heart rate less than 50 beats per minute for at least 24 hours) during hospitalization; (4) patients who were willing to participate in the study.
- Exclusion criteria: (1) Patients with a prior history of bradycardia; (2) patients not exhibiting sustained bradycardia during hospitalization; (3) patients who were unwilling to participate in the study.
3.3. Diagnostic Tests and Monitoring
We used PCR for SARS-CoV-2 and reverse-transcription polymerase chain reaction (RT-PCR) assays to detect SARS-CoV-2 RNA from nasopharyngeal swab specimens. All patients underwent the following examinations:
- Lung HRCT scans.
- Echocardiography.
- Daily electrocardiography.
- Examination of inflammatory markers such as C-reactive protein (CRP), ferritin, D-dimer, and blood serum electrolytes.
Non-contrast high-resolution CT chest imaging was performed on all patients. We assessed the severity of each patient’s condition using a scoring system based on the visual evaluation of each involved lobe. Specialist radiologists determined the severity of the involvement, categorizing it as mild, mild to moderate, moderate, or severe.
3.4. Patient Treatment and Follow-up
Patients were treated according to the Iranian national guidelines, which have been updated several times, with the most recent version being the sixth edition. All patients received various doses of corticosteroids, including dexamethasone and methylprednisolone. Sixteen patients were treated with remdesivir, based on their physician’s decision and clinical course. After evaluations, we followed up with patients regarding heart rate and mortality one month after discharge from the hospital.
3.5. Data Analysis
We used SPSS software to analyze the data and reported descriptive statistics for patients’ demographic features, clinical characteristics, and laboratory results in terms of numbers and relative frequency. Qualitative data were described using frequency, while quantitative data were presented as mean ± standard deviation.
3.6. Ethical Issues
Human rights were respected in accordance with the Helsinki Declaration of 1975, as revised in 1983. The Ethical Committee of Isfahan University of Medical Sciences (IR.MUI.MED.REC.1400.356) approved the study. Informed consent was obtained from the patients, and all ethical procedures were followed.
4. Results
In our study, we analyzed 50 patients with confirmed COVID-19, 26 of whom were male. The average age of the patients was 63.7 ± 19.3 years. The most common comorbidities among the patients were hypertension, diabetes mellitus, and ischemic heart disease. Among these patients, 44 tested positive for COVID-19 via PCR, while the remaining 6 were diagnosed based on clinical symptoms and typical HRCT lung scan findings suggestive of COVID-19. A total of 37 patients had moderate to severe pulmonary involvement, as determined by HRCT lung scans. Fifteen patients had an ejection fraction (EF) of less than 50% on echocardiography, and 20 patients had oxygen saturation levels of less than 85% in room air after hospitalization.
4.1. Statistical Significance Tests
We conducted various statistical significance tests to determine the relationship between different clinical parameters and mortality. The P-value is a measure of the probability that an observed difference could have occurred by random chance. Generally, a P-value of less than 0.05 is considered statistically significant (Table 2).
| Parameters | Minimum - Maximum | Mean ± SD | Significant Difference |
|---|---|---|---|
| Age (y) | 24.00 - 92.00 | 63.70 ± 19.30 | No |
| Troponin (ng/dL) | 0.90 - 7984.90 | 693.39 ± 1629.88 | Yes |
| D-dimer (ng/mL) | 45.70 - 8310.00 | 2075.75 ± 1915.28 | No |
| CRP at admission (mg/L) | 14.00 - 174.00 | 99.50 ± 46.75 | Yes |
| CRP at discharge (mg/L) | 1.00 - 148.00 | 31.72 ± 42.52 | Yes |
| Ferritin (ng/mL) | 41.00 - 1650.00 | 774.92 ± 479.16 | Yes |
| LDH (U/L) | 32.00 - 1721.00 | 884.68 ± 358.59 | Yes |
| CPK (U/L) | 34.00 - 23850.00 | 962.86 ± 3509.80 | No |
| Sodium (mmol/L) | 122.00 - 154.00 | 140.02 ± 4.45 | No |
| Potassium (mmol/L) | 3.20 - 7.00 | 4.73 ± 0.86 | No |
| Calcium (mg/dL) | 7.40 - 11.40 | 8.64 ± 0.68 | No |
| Phosphorus (mg/dL) | 2.30 - 4.70 | 3.21 ± 0.51 | No |
| pH (in VBG) | 7.06 - 7.50 | 7.30 ± 0.08 | No |
| PCO2 (in VBG) (mmHg) | 28.10 - 66.80 | 44.15 ± 9.76 | No |
| HCO3 (in VBG) (mmol/L) | 6.90 - 35.50 | 21.14 ± 4.89 | No |
| Oxygen saturation at admission (%) | 64.00 - 92.00 | 83.18 ± 7.12 | No |
| Oxygen saturation at discharge (%) | 73.00 - 99.00 | 92.28 ± 4.85 | Yes |
| EF (%) | 15.00 - 60.00 | 49.70 ± 10.17 | Yes |
| Temperature (°C) | 35.00 - 39.00 | 36.96 ± 0.68 | No |
| Mean blood pressure (mmHg) | 53.60 - 126.60 | 89.96 ± 15.39 | No |
| Heart rate at admission (bpm) | 18.00 - 50.00 | 29.12 ± 6.38 | No |
| Heart rate follow-up after 1 month (bpm) | 60.00 - 105.00 | 76.89 ± 10.28 | No |
| WBC count (cells/μL) | 2900.00 - 21300.00 | 7754.00 ± 4213.06 | No |
| Lymphocyte count (cells/μL) | 195.30 - 3296.70 | 1075.97 ± 613.89 | Yes |
| Hemoglobin (g/dL) | 9.50 - 16.10 | 12.68 ± 1.71 | No |
| Admission days | 2.00 - 47.00 | 10.54 ± 8.16 | No |
Mean, Standard Deviation, Minimum, and Maximum Levels of Laboratory Parameters in the Studied Patients
- Troponin: The mean troponin level was significantly higher in patients who died compared to survivors (P = 0.018). This indicates that elevated troponin levels are associated with higher mortality.
- Ferritin: Ferritin levels were significantly higher in patients who died (P = 0.001), suggesting that higher ferritin levels are linked to increased mortality.
- Lactate dehydrogenase (LDH): Patients who died had significantly higher LDH levels compared to survivors (P = 0.002), indicating that elevated LDH levels are associated with higher mortality.
- Lymphocyte count: There was a significant reduction in lymphocyte count in patients who died compared to survivors (P = 0.001), suggesting that lymphopenia is associated with higher mortality.
- Oxygen saturation at discharge: Lower oxygen saturation at discharge was significantly associated with higher mortality (P = 0.003).
- C-reactive protein at admission: Higher CRP levels at admission were significantly associated with increased mortality (P = 0.022).
- C-reactive protein at discharge: The CRP levels at discharge were significantly higher in patients who died (P = 0.001).
These results indicate that several clinical parameters, including elevated troponin, ferritin, and LDH levels, lymphopenia, lower oxygen saturation at discharge, and higher CRP levels at both admission and discharge, are significantly associated with increased mortality in COVID-19 patients with bradycardia.
4.2. Evaluation of Heart Rate Measurements in Predicting Patient Mortality Using ROC Curve Analysis
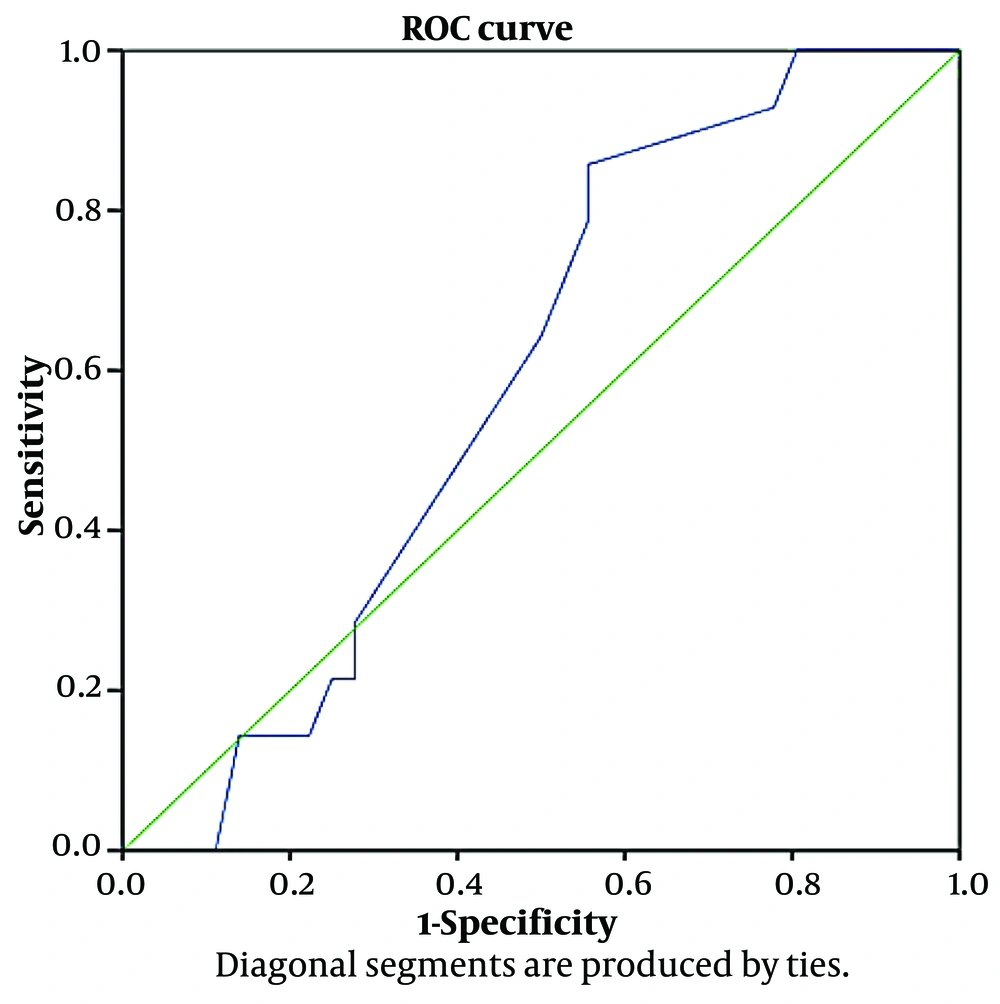
From July 22, 2020, to January 19, 2021, 6,031 clinically suspected COVID-19 patients were admitted to Al-Zahra Hospital, of whom 1,139 died in the hospital. This corresponds to an 18.8% mortality rate during this period. In our study group of patients with bradycardia, the mortality rate was 28%, which is significantly higher than the overall rate. The average heart rate of the patients was 29.12 ± 6.38 beats per minute. We divided the heart rates of patients, ranging from 17 to 51 beats per minute, into regular intervals and determined the mortality rate in each heart rate interval (Table 3). We then examined the sensitivity and specificity of different heart rates in predicting mortality. A decrease in heart rate could predict death with greater sensitivity but less specificity. We concluded that heart rate was not specific enough to predict mortality.
| Positive if Greater than or Equal To | Sensitivity (%) | 1 - Specificity (%) |
|---|---|---|
| 17.0 | 100 | 100 |
| 19.5 | 100 | 92 |
| 21.5 | 100 | 86 |
| 22.5 | 100 | 83 |
| 23.5 | 100 | 81 |
| 24.5 | 93 | 78 |
| 25.5 | 86 | 56 |
| 26.5 | 79 | 56 |
| 27.5 | 71 | 53 |
| 29.0 | 64 | 50 |
| 30.5 | 29 | 28 |
| 31.5 | 21 | 28 |
| 32.5 | 21 | 25 |
| 33.5 | 14 | 22 |
| 35.0 | 14 | 17 |
| 36.5 | 14 | 14 |
| 37.5 | 0 | 11 |
| 40.0 | 0 | 8 |
| 46.0 | 0 | 3 |
| 51.0 | 0 | 0 |
Frequency and Mortality Rate in Different Heart Rate Groups
ROC curve analysis was used to evaluate the predictive power of heart rate measurements for patient mortality. The ROC curve plots the true positive rate (sensitivity) against the false positive rate (1-specificity) at various thresholds. The area under the curve (AUC) measures the model's ability to distinguish between survival and mortality. An AUC of 0.587 indicates limited predictive power, suggesting that the results may not be reliable. Figure 1 shows the ROC curves evaluating heart rate measurements at hospitalization for predicting mortality. The AUC of 0.587 further indicates limited predictive power.
4.3. Follow-up and Outcomes
All discharged patients were followed up by telephone for one month. Four patients died during this follow-up period. Three of these patients had troponin levels > 2000 at the time of hospitalization, an EF < 50%, were treated for acute coronary syndrome (ACS), and had low oxygen saturation after discharge. One patient died suddenly of unknown causes. No cases of bradycardia were reported within one month after discharge.
5. Discussion
Our research detailed a case series of hospitalized COVID-19 patients who experienced severe, sustained bradycardia during their hospital stay. We observed a 28% mortality rate both early during hospitalization and after discharge, but this was not directly linked to cardiovascular events such as heart failure, arrhythmia, ischemia, or myocardial infarction. The primary causes of death were respiratory failure and sepsis syndrome, leading to multi-organ involvement. This suggests that while bradycardia was associated with death, it was not the direct cause. Instead, bradycardia could serve as a prognostic marker for severe disease, especially in ICU patients. A global survey by electrophysiologists (8) indicated that atrial fibrillation was the most common tachyarrhythmia, while sinus bradycardia and complete heart block were the most prevalent bradyarrhythmias. It is suggested that sinus bradycardia might serve as a clinical indicator of COVID-19 in its mild to moderate forms, resolving within 24 to 48 hours without poor prognosis (9). In our study, bradycardia persisted for up to a week during hospitalization. Despite electrolyte abnormalities resolving, bradycardia persisted in 41 patients. Four patients (8%) developed complete heart block, necessitating pacemaker implantation, similar to the findings of (8). Troponin levels were significantly associated with mortality, consistent with other studies indicating elevated troponin as a risk factor for severe disease, ICU admissions, and higher mortality (1). Elevated troponin in COVID-19 patients could result from myocardial injury, myocarditis, coronary microvascular ischemia, pulmonary embolism, decompensated heart failure, arrhythmia, hypoxemia, and hypotension. Although systemic inflammation can increase the risk of type 1 myocardial infarction, no significant increase in ST-segment elevation myocardial infarction (STEMI) risk was reported in COVID-19 patients. Ejection fraction was significantly associated with mortality, although prior cardiac output data were lacking. Other studies have shown new-onset heart failure in end-stage COVID-19, potentially due to cytokine storm syndrome. Inflammatory markers such as CRP, procalcitonin, and leukocytes were significantly elevated. Each unit increase in CRP raised the mortality risk by 2%, suggesting a link between inflammation and cardiac complications. Lymphopenia was also significantly associated with mortality.
A meta-analysis by Umeh et al. (9) linked lymphopenia to poor outcomes in severe COVID-19, potentially due to increased pro-inflammatory cytokines, especially IL-6. Lymphocytes expressing ACE2 may be direct targets of SARS-CoV-2. Hypoxia, resulting from lung injury and respiratory failure, was associated with arrhythmia. Hypoxia activates anaerobic glycolysis, reducing intracellular pH and increasing extracellular potassium and cytosolic calcium, which leads to alterations in action potential duration. Admission oxygen saturation was 83.18 ± 7.12, and discharge oxygen saturation was 92.28 ± 4.85. Discharge oxygen saturation was significantly associated with mortality. It is reported bradycardia in COVID-19 patients related to remdesivir administration (10). However, in our study, bradycardia was observed in patients both receiving and not receiving remdesivir, with some developing bradycardia before starting the medication.
5.1. Limitations
The small sample size was the primary limitation of this study, making it difficult to obtain precise results regarding parameter relationships. We conducted follow-up only via telephone interviews after one month. Long-term follow-up with close observation is necessary to better understand mortality rates, especially sudden death and the cardiovascular impacts of COVID-19. Seventeen patients were on heart rate-affecting medications, but none had a history of bradycardia. The different treatments for various complications may have introduced bias, necessitating future clinical trials to confirm these results. The therapeutic challenges posed by the co-occurrence of COVID-19 and cardiovascular complications require further study (11).
5.2. Conclusions
Our study detailed the clinical course of COVID-19 patients who experienced sustained bradycardia during hospitalization. While we observed an association between bradycardia and increased mortality, our study design does not allow for a definitive comparison between patients with and without bradycardia. Therefore, we cannot conclusively state that bradycardia is an independent prognostic marker for mortality in COVID-19 patients. Future research should design case-control studies to better understand the prognostic significance of bradycardia. Despite these limitations, our findings suggest that bradycardia in COVID-19 patients may indicate severe disease, particularly in ICU settings. We recommend that clinicians closely monitor bradycardia as a potential marker for disease severity. Further studies are needed to explore the impact of bradycardia on COVID-19 outcomes, considering other comorbidities and the effects of various medications.