1. Background
Peripheral artery disease (PAD) is a condition characterized by the complete or incomplete occlusion of non-cardiac, non-intracranial peripheral arteries in the upper and lower extremities, which may result in reduced blood flow or tissue damage. It is usually the result of atherosclerosis of the vessel wall but may also be due to embolism, thrombosis, fibromuscular dysplasia, or vasculitis. PAD is a common complication of type 2 diabetes mellitus (T2DM) (1). The risk of developing PAD correlates with known cardiovascular risk factors, such as hypertension, dyslipidemia, smoking, and DM (2-5). The prevalence of PAD is two to sevenfold higher in diabetic patients compared to the average population, ranging from 9 to 55% in these patients (6-8). The PAD in the lower limbs significantly increases morbidity and disease burden in diabetic patients and may lead to major adverse limb events (MALEs) such as amputations, which often lead to physical disability and emotional disturbances. Although diabetic neuropathy is widely known as the major cause of diabetic limb lesions and amputation, PAD is also responsible for these sequelae (9-13).
The diagnostic methods for PAD are based on clinical signs and symptoms. Evaluation of symptoms can be handled with available questionnaires for limb pain, and signs are assessed by Ankle-Brachial Index (ABI), toe Brachial Index (TBI), and arterial Doppler ultrasound. Many factors may interfere with the accuracy of these methods. For instance, PAD is asymptomatic in most patients. Also, concomitant diabetic peripheral neuropathy may affect the perception of pain. Therefore, the presence of intermittent claudication (IC) and the absence of peripheral pulses are deemed insufficient diagnostic indicators for PAD (6, 7).
Aspirin (ASA) is the most commonly used antiplatelet medication in chronic stable PAD patients. However, its efficacy is challenged due to high rates of MALEs unaffected by it (14, 15). To enhance its efficacy, many trials have been conducted to suggest the addition of an agent. Rivaroxaban is an example of these agents, evaluated in two notable trials, COMPASS and ATLAS TIMI-51 (16, 17), showing promising outcomes in reducing MALEs and major adverse cardiac outcomes (MACEs) with ASA and rivaroxaban dual therapy rather than ASA monotherapy (16). Rivaroxaban is a direct factor Xa inhibitor that has been shown to cause a lower bleeding tendency compared to vitamin K antagonists in atrial fibrillation (AF) and venous thromboembolism (18, 19). Additionally, a recent study has demonstrated the potential of low-dose rivaroxaban to overcome ASA non-sensitivity in PAD patients (20). Nonetheless, the effects of the combination of ASA and rivaroxaban on the clinical presentations of PAD before revascularization have not been established (21).
2. Objectives
In this study, we aim to investigate the effect of ASA with and without rivaroxaban on symptomatic peripheral vascular disease of the lower limbs in diabetic patients prior to revascularization.
3. Methods
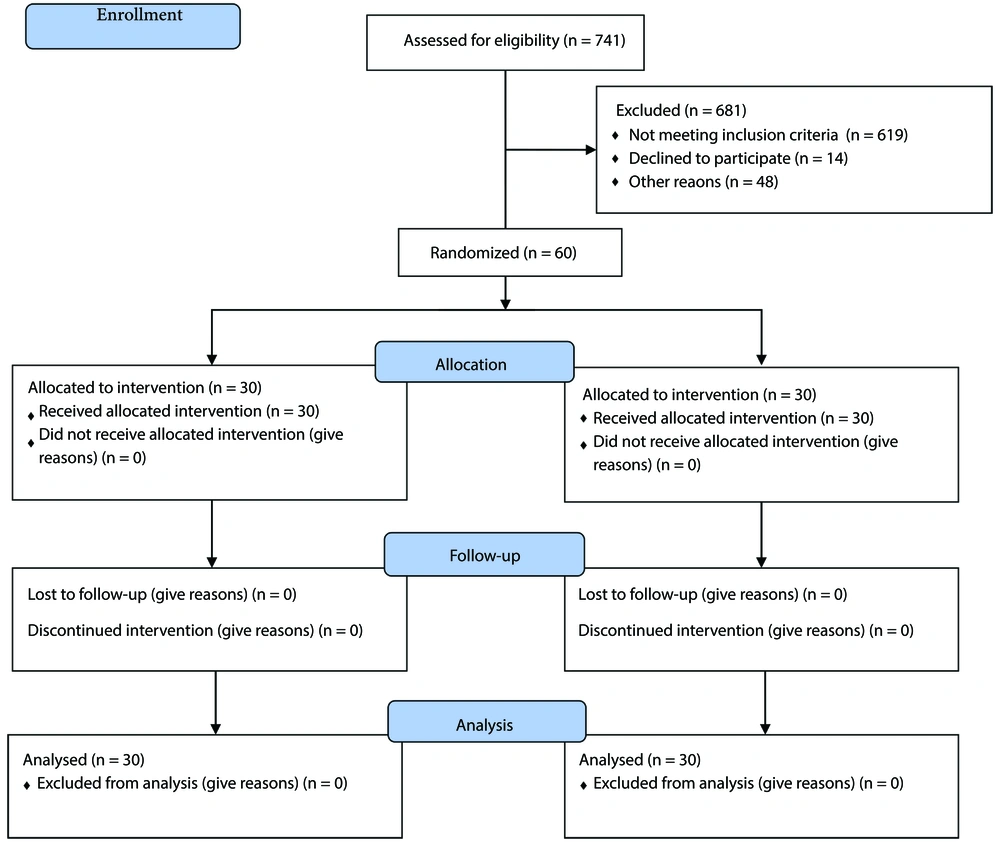
The current study is an open-label randomized controlled trial designed to compare the effects of ASA and rivaroxaban dual therapy to ASA monotherapy in diabetic PAD patients. All eligible patients referred to Loghman Hospital Clinic, Tehran, Iran, between January and August 2023 were included in this study. A total of sixty patients were included. Patients were randomized electronically in a 1:1 ratio using CRF (RedCap version 11.0.3), and randomization was balanced using randomly swapped blocks. The randomization list was created using software (RedCap) with blocks of variable sizes. The study flow is presented in Figure 1, which is a consolidated standards of reporting trials (CONSORT) flow diagram of the study.
The inclusion criteria were: patients with T2DM (diagnosed by one of the following: Fasting Blood Sugar ≥ 126 mg/dL, 2-hour postprandial blood sugar > 180 mg/dL, or hemoglobin A1C > 6.5%) who have symptomatic PAD of the lower limbs presenting with one of the following symptoms according to the current guidelines (18, 19): IC, limb pain at rest or overnight, and PAD-related skin changes that do not require invasive management.
Exclusion criteria were: Uncontrolled DM with hemoglobin A1C ≥ 7.5%, history of revascularization, known joint disorder, history of severe renal impairment, history of any nontraumatic bleeding, history of coagulation disorders, systemic treatment with CYP3A4 inhibitors/inducers, concomitant treatment with other anticoagulants, and known hypersensitivity to rivaroxaban or ASA.
3.1. Drug Administration and Follow Up
Patients were randomly divided into two groups using a computer-generated randomization schedule prepared before the study. In group A, patients received rivaroxaban 2.5 mg twice a day and ASA 100 mg daily, while in group B, patients received ASA 100 mg daily (initiated or continued at randomization) for 12 weeks. Follow-up was performed by primary care physicians at regular office visits every 4 weeks. Proper administration of the medication was emphasized, and patients who did not complete the medication or had drug adherence less than 80% were considered non-adherent and were excluded. At the end of the twelfth week, the patients were interviewed and evaluated.
3.2. Assessment of the Outcomes
The evaluation of IC was assessed through clinical history and physical activity tests and reported based on the Fontaine Classification (Table 1) (22). Stages III and IV are considered severe IC.
| Grades | Symptoms |
|---|---|
| Stage I | Asymptomatic, incomplete blood vessel obstruction |
| Stage II | Mild claudication pain in the limb |
| IIA | Claudication at a distance > 200 m |
| IIB | Claudication at a distance < 200 m |
| Stage III | Rest pain, mostly in the feet |
| Stage IV | Necrosis and/or gangrene of the limb |
Another evaluated symptom was rest pain in the lower extremity, which usually occurs overnight. During history taking, we asked the patients if they experienced the symptom at rest or overnight during each visiting session and simply recorded it as “yes” or “no”. The differentiation between arterial rest pain and neuropathic rest pain was performed by the interviewing physician.
Another evaluated outcome was the ABI. The ABI was measured by a trained physician before and after the treatment. Brachial systolic blood pressure (bSBP) was measured using BP cuffs, and ankle systolic blood pressure (aSBP) was measured by a portable Doppler ultrasound unit. Measured ABIs were categorized into four stages of severity: An ABI less than 0.4 was considered severe PAD, an ABI between 0.4 and 0.8 was considered moderate, an ABI between 0.8 and 1 was considered mild, and an ABI between 1 and 1.4 was considered normal (23).
3.3. Statistical Analyses
By choosing a confidence level of 95%, a power of 80%, and an effect size of 0.36 (medium effect size in Cohen's equation), and using G*Power 3.1.9.4 software (24), the sample size was calculated to be 60 people (thirty people in each group). Mean, standard deviation, frequency, and percentage were used to describe the data to provide an intergroup analysis at the same stages for each test. The Wilcoxon test was used to compare values before and after treatment in each group, and chi-square and Fisher Exact tests were used to compare between groups. Analyses were performed using SPSS 25.0 statistical software. A P-value less than 0.05 was considered statistically significant.
3.4. Ethical Considerations
This study was performed in accordance with the Declaration of Helsinki and was approved by the Shahid Beheshti Research Ethics Board. All patients provided verbal and written informed consent before participating in the study and were allowed to leave the study at any time of their own will.
4. Results
Sixty patients were enrolled in our study, divided into two groups, each containing thirty patients. During the study, none of the patients were excluded, and no drug-related adverse events, such as bleeding, occurred. The baseline characteristics of the patients in each group are presented in Table 2. Since the P-value for every characteristic is calculated to be greater than 0.05, there is no statistically significant difference in the baseline features of the two groups.
| Variables | Groups | P-Value | |
|---|---|---|---|
| ASA alone | Rivaroxaban + ASA | ||
| Age | 0.87 | ||
| 45 - 55 | 5 (16.7) | 7 (23.3) | |
| 55 - 65 | 10 (33.3) | 11 (36.7) | |
| 65 - 75 | 10 (33.3) | 8 (26.7) | |
| > 75 | 5 (16.7) | 4 (13.3) | |
| Gender | 0.302 | ||
| Male | 17 (56.7) | 13 (43.3) | |
| Female | 13 (43.3) | 17 (56.7) | |
| Smoker | 0.095 | ||
| No | 22 (73.3) | 27 (90.0) | |
| Yes | 8 (26.7) | 3 (10.0) | |
| BMI | 0.448 | ||
| Normal | 3 (10.0) | 5 (16.7) | |
| High | 27 (90.0) | 25 (83.3) | |
Abbreviation: ASA, aspirin.
a Values are expressed as No. (%).
b P-value based on chi-square and Fisher Exact test.
The rates of IC before and after treatment are demonstrated in Table 3. Before the initiation of treatment, all patients in both groups had IC, and there was no statistically significant difference between the two groups (P = 0.857). After the treatment, both groups demonstrated symptom relief, as the number of patients with stage I and II claudication increased and stage III and IV decreased (P-value < 0.001 in both groups). The comparison of the two groups after treatment implied that symptom progression is more likely to be reverted in patients undergoing the combination therapy (P-value = 0.011). Interestingly, combination therapy treated all the stage IV patients, who had skin changes such as small ulcers and localized blackened skin (early symptoms of necrosis).
Abbreviation: ASA, aspirin.
a Values are expressed as No. (%).
b P-value based on Fisher Exact test.
c P-value based on Wilcoxon signed ranks test.
The rates of lower extremity pain at rest or overnight before and after treatment are shown in Table 4. Prior to treatment, the prevalence of leg pain at rest/overnight was equal in both groups (P = 0.612). After treatment, both groups showed a reduction in symptomatic patients (P-value < 0.05 in both groups). Moreover, patients receiving combination therapy had significantly lower rates of nocturnal or rest pain compared to those on ASA monotherapy (P = 0.009).
Abbreviation: ASA, aspirin.
a Values are expressed as No. (%).
b P-value based on Fisher Exact test.
c P-value based on Wilcoxon signed ranks test.
Ankle-Brachial Index was measured in patients of both groups before and after treatment, and the results are presented in Table 5. Prior to the treatments, there was no meaningful difference between the groups in each category (P = 0.186). After treatment, ABI improved with both regimens (P < 0.05 in both groups). Combination therapy had better outcomes as it normalized ABI in six patients, compared to none in the ASA monotherapy group, and resolved all the severe indices; whereas seven patients in the other group remained with severe ABI (P = 0.001).
Abbreviation: ASA, aspirin; ABI, Ankle-Brachial Index.
a Values are expressed as No. (%).
b P-value based on Fisher Exact test.
c P-value based on Wilcoxon signed ranks test.
5. Discussion
This study was conducted as a randomized clinical trial to compare the efficacy of ASA alone versus the combination of ASA and rivaroxaban in alleviating the signs and symptoms of PAD in patients with controlled diabetes mellitus (DM). Sixty patients were included in the study and randomly divided into two groups, each containing thirty patients. Prior to the initiation of the treatment, baseline features of the patients in the groups were compared, demonstrating no significant difference in age, gender, BMI, and smoking status. Additionally, the patients in the two groups had similar comorbidities and were receiving similar standard medications such as statins. Moreover, the symptoms of PAD were similarly frequent in the groups, and patients had not received any prior treatment for their PAD.
After the treatment, the patients receiving the combination of rivaroxaban and ASA showed significant improvement in IC and limb pain at rest. Additionally, ABI was normalized in more patients in this group compared to those receiving ASA alone. IC has been a major concern in our study. Currently, the only FDA-approved medications for IC are pentoxifylline and cilostazol. Cilostazol is more accepted because it improves walking distance, despite its adverse effects. However, its efficacy on MACE or major adverse limb events (MALE) remains uncertain (25-27). High-quality evidence supports a stronger role for surgery, endovascular intervention, and exercise in the treatment of IC rather than the current medical management (28). Many of these management strategies are not indicated in the early stages of PAD, leading to the ongoing struggle to establish a medical approach for PAD.
The beneficial role of ASA in the management of PAD is established in several studies (29). Several studies have proposed medical strategies to aid ASA, like the addition of clopidogrel, for the treatment of DM-related macrovascular complications (30-32). The proposed medications proved effective; however, they were mostly accompanied by bleeding side effects. Through these investigations, rivaroxaban showed promising outcomes. Rivaroxaban is a selective direct factor Xa inhibitor that is widely prescribed for the prevention and treatment of venous thromboembolism (VTE). It is also approved for the prevention of thrombotic events caused by atrial fibrillation (AF) (33). Rivaroxaban, as a direct factor Xa inhibitor, causes a significantly lower bleeding tendency compared to vitamin K antagonists (18, 19). A meta-analysis showed that rivaroxaban is superior to ASA and warfarin for PAD, regarding its lower incidence of MALEs and major bleeding (34).
The rationale for the evaluation of rivaroxaban and other anticoagulation therapies in the treatment of IC arises from the pathophysiology of IC and PAD. Vascular atherosclerosis is a common sequel of DM leading to arterial obstruction and tissue ischemia, which accounts for the symptoms of PAD alongside inflammation, reduced microvascular flow, and impaired angiogenesis (35). Moreover, thrombosis is a common finding in histopathologic examinations of amputated tissues (36). This emphasized role of clot in the pathogenesis of PAD is the pivotal reason for these studies and warrants the effort to resolve it so that symptoms can be alleviated.
The combination of rivaroxaban and ASA has been compared to ASA monotherapy in a few studies. The ATLAS TIMI-51 trial evaluated the efficacy of these regimens in reducing MACE. It concluded that rivaroxaban was superior to placebo when prescribed along with ASA; however, this superiority came at the cost of a substantially increased risk of bleeding (17). The COMPASS trial indicated a significant reduction in MACE after adding rivaroxaban at a dosage of 2.5 mg every 12 hours to ASA at a daily dosage of 100 mg. In this trial, major adverse limb events (MALE), including acute and chronic limb ischemia and amputation due to PAD, were also evaluated. They found that the combination therapy reduces MALE. However, the addition of rivaroxaban led to an increase in major bleeding events, mostly from a gastrointestinal source, but the results showed no increase in life-threatening bleeding. The overall analysis of this study, considering MACE and MALE, found a favorable benefit with the combination of rivaroxaban and ASA (37). A sub-analysis of the COMPASS trial suggested that the estimated net clinical benefit of combination therapy is 3.2% (95% CI, 0.6% - 5.3%) (38).
In another randomized placebo-controlled trial, the administration of rivaroxaban reduced MALEs, either with or without ASA. Similarly, they found that rivaroxaban increased the incidence of major bleedings, with no increase in fatal, life-threatening bleedings (21). Another study reported similar results, but no difference was found between the rivaroxaban group and the placebo group in the incidence of major bleeding, assessed according to the Thrombolysis in Myocardial Infarction (TIMI) classification (39). Some other studies reported similar outcomes, supporting the net benefit of rivaroxaban and ASA dual therapy (40-46). For instance, the VOYAGER PAD trial has supported the role of rivaroxaban and ASA dual therapy in reducing the risk of acute limb ischemia (47). The RIVAL-PAD trial showed significant improvement in post-intervention ABI with rivaroxaban and ASA compared to clopidogrel and ASA. However, it found no difference between the two regimens in baseline ABI and chronic total occlusion (48). Another study comparing the efficacy of rivaroxaban and clopidogrel, in combination with ASA, showed the same results; rivaroxaban was superior in above-the-knee PAD. However, they had no significant difference in below-the-knee PAD, and rivaroxaban was associated with a higher bleeding incidence (49).
Most of these studies mainly focus on MALEs and the bleeding caused by regimens. However, we decided to focus on the potency of the regimens for symptom relief, due to the lack of evidence in this area. Moreover, unlike most of the mentioned studies, our study was conducted on patients who underwent no revascularization therapy, and we aimed to evaluate the ameliorative effect of rivaroxaban early in the course of treatment and before revascularization.
Our study followed only sixty patients for 12 weeks. It is a relatively short period of follow-up and involves small groups of patients, despite possessing an efficiently calculated sample size. In order to apply our results in future clinical practice, it is crucial to examine them on a larger scale. The addition of rivaroxaban to ASA is deemed a promising treatment in diabetic patients with PAD, and its prescription may herald a novel, life-saving intervention. Thus, it is recommended that further investigations be conducted with a larger sample size and for a longer duration. Moreover, it is recommended that other variables such as mortality, need for revascularization, and rates of amputation be regarded. Our study included only diabetic patients, and we recommend further investigations including non-diabetic PAD patients.
5.1. Conclusions
Our findings suggest that the combination of rivaroxaban and ASA is more potent than ASA alone for the treatment of PAD symptoms before any surgical management. It reduced IC and leg pain at rest, and it significantly improved the ABI. Additionally, the combination of rivaroxaban and ASA did not increase the risk of bleeding compared to ASA alone. There were no major adverse limb events in our study.