1. Background
Congenital heart disease (CHD) is a leading cause of mortality among children, often exposing them to numerous risk factors that can contribute to kidney disease later in life. Pathophysiological changes associated with an abnormal heart and the presence of microalbuminuria, recognized as a reliable predictor of advanced renal disease, are among the prominent contributors to this risk (1, 2). Heart failure, as studies have shown, plays a critical role in disrupting renal circulation (3). This disruption is compounded by factors such as polycythemia, chronic cyanosis, hypoxia, altered renal blood flow, and impaired neurohormonal activation, all of which create a complex interplay of pathological changes that exacerbate kidney dysfunction in CHD patients and contribute to the development of comorbid chronic kidney disease (CKD) (1, 3). Cyanotic nephropathy (CN) affects approximately 30% - 50% of children with cyanotic CHD, causing significant damage to renal tubular function, particularly during the first decade of life. In addition to harming the tubules, CN disrupts glomerular function, leading to complications such as proteinuria, nephrotic syndrome, reduced glomerular filtration rate (GFR), and azotemia (2). Reduced cardiac output in CHD further strains kidney function, increasing the risk of developing CKD (4). This risk is heightened by a range of factors, some of which are irreversible. These include ischemic injury during cardiopulmonary failure in surgeries, hyperviscosity caused by uncorrected cyanotic heart disease, damage to the glomeruli and lungs, and renal or urinary neoplasms linked to syndromic CHD (5). Chronic cyanotic congenital heart disease (CCHD) leads to systemic hypoxia, including in the kidneys, where it triggers structural and functional changes in the glomeruli. These changes include glomerular hypertrophy, thickening of capillary walls, mesangial expansion, and both focal and global glomerulosclerosis, all of which contribute to kidney dysfunction (6, 7). Gupte et al. (8) highlighted these structural abnormalities in their study, noting findings such as proliferative atherosclerosis, interstitial fibrosis, and renal corpuscle changes in kidney autopsies of children with CCHD compared to age-matched controls. Similarly, research by Fang et al. (5) revealed that nearly half of children with CHD have kidney-related conditions, with CCHD patients being almost twice as likely to develop clinically significant CKD compared to those with acyanotic congenital heart disease (aCCHD).
2. Objectives
Although there is increasing evidence of significant kidney involvement in children with CHD, important gaps remain in our understanding of their renal outcomes. Currently, no standardized guidelines exist for assessing kidney function in CHD patients, leaving a critical void in clinical care. This study seeks to address this issue by comparing the degree of renal involvement among children with CCHD, aCCHD, and healthy controls, using indicators such as microalbuminuria and GFR. By consolidating current knowledge and identifying key predictors, this research aims to contribute to the development of effective prevention strategies and personalized renal monitoring protocols for pediatric CHD patients.
3. Methods
This case-control study was conducted on 160 children, consisting of 110 children with CHD and 50 controls. The CHD children were divided into CCHD and aCCHD groups, with 60 and 50 children, respectively. The study was conducted at the Pediatric Cardiology Unit of the Pediatrics Department, Ali Ibn Abitalib Hospital, Zahedan University of Medical Sciences, Zahedan, Iran. The hospital is a major referral center for pediatric cardiac cases in the southeastern region of Iran and the neighboring countries of Afghanistan and Pakistan. The study was conducted between January 1, 2022, and October 31, 2023. Patients were selected from among CHD children and then divided into cyanotic and acyanotic groups. Controls were selected from children referred to the hospital for routine or annual checkups.
3.1. Sampling Methods and Sample Size
This study utilized a stratified sampling approach to ensure adequate representation of the target groups: Children with CHD (CCHD and aCCHD) and healthy controls. The sample size of 160 participants was determined based on a power analysis to achieve reliable and statistically significant results. The target population included 110 children diagnosed with CHD (60 with CCHD and 50 with aCCHD and 50 control children. The larger number of CHD patients compared to controls reflects the study's primary focus on analyzing renal involvement within the CHD population, particularly comparing the cyanotic and acyanotic subgroups. This imbalance allows for a more in-depth exploration of differences between CCHD and aCCHD groups, which is critical for understanding the varying impacts of cyanosis on renal function. Control participants were included to establish baseline renal parameters for comparison. These children were selected from those referred to the hospital for routine or annual checkups, ensuring they were free from CHD and other conditions known to affect renal function. The control group size, though smaller, was sufficient for comparison purposes, as the study's main objective was to investigate differences within the CHD cohort. This sampling strategy ensured that the study adequately captured variability within the CHD population while maintaining a robust control group for meaningful comparisons.
3.2. Criteria
Children less than 18 years of age with an echocardiographic diagnosis of CHD who provided informed written consent were included. Exclusion criteria were children with corrective surgery, febrile illnesses within 2 weeks prior to and at the time of the study, symptoms of urinary tract infection such as dysuria, frequency, urethral discharge, use of antibiotics and nonsteroidal analgesics two weeks prior to the study, menstruating females, any other comorbidity known to affect renal function such as sickle cell disease, elevated blood pressure, and those who had taken drugs known to affect protein excretion, such as radio-opaque dye and nitrofurantoin, within the previous 4 weeks. Children with diabetes, all heart diseases except CHD, obesity, a family history of kidney diseases, and inherited kidney disorders were excluded from the study.
3.3. Microalbuminuria
Microalbuminuria was measured quantitatively in the urine by immunoturbidometry using an ELISA technique. The albumin concentration in the urine sample is proportional to the turbidity, which was analyzed by a microplate reader. Results of microalbuminuria were reported as positive if the spot urine microalbumin/urine creatinine ratio was > 30 mg/mg creatinine.
3.4. Estimated Glomerular Filtration Rate
In children, the most frequently used creatinine-based estimated glomerular filtration rate (eGFR) equation is the revised bedside Schwartz formula:
This is recommended by the national kidney disease education program for use with creatinine methods with calibration traceable to IDMS. The
3.5. Weight and Height Measurements
For each participant, weight and height were measured as part of the data collection process. Weight was measured using a calibrated digital scale, ensuring accuracy to the nearest 0.1 kg. Participants were weighed wearing light clothing and no shoes. Height was measured using a stadiometer, recorded to the nearest 0.1 cm. For younger children unable to stand, a recumbent length board was used. These measurements were critical for calculating the eGFR using the Schwartz equation, which relies on height as a key variable.
3.6. Instruments and Equipment
(1) Digital scale: Calibrated to measure weight accurately to 0.1 kg.
(2) Stadiometer: Used for standing height measurement, with precision to 0.1 cm.
(3) Recumbent length board: For measuring the length of infants and younger children who could not stand.
(4) ELISA microplate reader: For quantifying microalbuminuria via immunoturbidometry, ensuring precise detection of urine albumin levels.
(5) Urine collection containers: Sterile containers were used to collect urine samples, minimizing contamination.
(6) Creatinine analyzer: Calibrated to IDMS standards for measuring serum creatinine levels used in eGFR calculation.
These tools were carefully chosen and calibrated to ensure data accuracy and reliability in assessing renal function and growth parameters in the study participants.
3.7. Ethics
This study was approved by the Research Ethics Board of the Research Deputy, Zahedan University of Medical Sciences, Zahedan, Iran, and conducted in compliance with the Declaration of Helsinki. Informed consent was obtained from the parents or legal guardians of all participating children, ensuring their understanding and agreement to the study procedures. The study was registered under the ethical approval code IR.ZAUMS.REC.1398.378.
3.8. Statistical Analysis
All statistical analyses were performed using SPSS version 20 (SPSS Inc, Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation and compared using one-way analysis of variance or Kruskal-Wallis test, respectively, for normally and non-normally distributed data. Univariate logistic regression was then performed. Tukey or Dunn follow-up tests were performed for pairwise comparison of significant variables. The results of the comparison were expressed as P-values. Statistical significance was set at P < 0.05.
4. Results
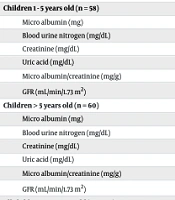
Height and weight were significantly reduced in children with CCHD across all age groups (P = 0.05), indicating a consistent pattern of growth impairment in this cohort. Table 1 presents the normal distribution of the measured variables in this study, with the exception of GFR, which exhibited a normal distribution. Table 2 outlines the variations in weight, height, and laboratory test results across the participant groups. In the overall cohort, children with CCHD showed significantly lower height and weight compared to the other groups (P = 0.05). Microalbuminuria levels were higher in children with CCHD (14.51 ± 7.81 mg/mg) compared to those with aCCHD (10.20 ± 1.95 mg/mg) and controls (10.31 ± 1.73 mg/mg) (P = 0.015). Uric acid was significantly elevated in children with CCHD (5.26 ± 1.35 mg/dL) compared to both aCCHD (3.48 ± 0.8 mg/dL) and controls (3.62 ± 0.77 mg/dL) (P < 0.001). The albumin/creatinine ratio was also increased in children with CCHD (26.59 ± 14.15 mg/g), followed by aCCHD (20.57 ± 6.26 mg/g) and controls (20.47 ± 5.63 mg/g) (P = 0.023). The GFR was significantly lower in CCHD children (85.15 ± 19 mL/min/1.73 m2) compared to both aCCHD (102.61 ± 14.91 mL/min/1.73 m2) and the control group (101.64 ± 18.38 mL/min/1.73 m2) (P < 0.001).
| Variables | Mean ± SD | KS | P-Value |
|---|---|---|---|
| 1 year old children (n = 42) | |||
| Micro albumin (mg) | 10.69 ± 2.80 | 0.22 | < 0.001 |
| Blood urine nitrogen (mg/dL) | 10.57 ± 2.45 | 0.24 | < 0.001 |
| Creatinine (mg/dL) | 0.42 ± 0.06 | 0.33 | < 0.001 |
| Uric acid (mg/dL) | 3.99 ± 1.34 | 0.18 | 0.001 |
| Micro albumin/creatinine (mg/g) | 25.63 ± 6.70 | 0.20 | < 0.001 |
| GFR (mL/min/1.73 m2) | 75.38 ± 12.75 | 0.12 | 0.112 |
| Children 1 - 5 years old (n = 58) | |||
| Micro albumin (mg) | 11.52 ± 5.02 | 0.26 | < 0.001 |
| Blood urine nitrogen (mg/dL) | 12.69 ± 2.90 | 0.23 | < 0.001 |
| Creatinine (mg/dL) | 0.50 ± 0.07 | 0.22 | < 0.001 |
| Uric acid (mg/dL) | 4.05 ± 1.34 | 0.18 | < 0.001 |
| Micro albumin/creatinine (mg/g) | 23.40 ± 10.07 | 0.16 | 0.001 |
| GFR (mL/min/1.73 m2) | 100.26 ± 15.18 | 0.08 | 0.2 |
| Children > 5 years old (n = 60) | |||
| Micro albumin (mg) | 12.99 ± 6.77 | 0.27 | < 0.001 |
| Blood urine nitrogen (mg/dL) | 15.18 ± 3.20 | 0.13 | 0.017 |
| Creatinine (mg/dL) | 0.66 ± 0.10 | 0.23 | < 0.001 |
| Uric acid (mg/dL) | 4.47 ± 1.27 | 0.14 | 0.003 |
| Micro albumin/creatinine (mg/g) | 20.23 ± 11.87 | 0.19 | < 0.001 |
| GFR (mL/min/1.73 m2) | 105.67 ± 12.61 | 0.10 | 0.2 |
| All children 1 - 15 years old (n = 160) | |||
| Micro albumin (mg) | 11.85 ± 5.38 | 0.28 | < 0.001 |
| Blood urine nitrogen (mg/dL) | 13.07 ± 3.43 | 0.13 | < 0.001 |
| Creatinine (mg/dL) | 0.54 ± 0.13 | 0.18 | < 0.001 |
| Uric acid (mg/dL) | 4.19 ± 1.32 | 0.15 | < 0.001 |
| Micro albumin/creatinine (mg/g) | 22.80 ± 10.24 | 0.16 | < 0.001 |
| GFR (mL/min/1.73 m2) | 95.76 ± 18.38 | 0.08 | 0.014 |
Abbreviation: GFR, glomerular filtration rate.
| Variables and Participants | N | Mean ± SD | Test Value | P-Value |
|---|---|---|---|---|
| All Participants | ||||
| Weight (kg) | 16.969 | < 0.001 | ||
| Cyanotic | 60 | 13.13 ± 8.68 | ||
| Acyanotic | 50 | 18.00 ± 10.26 | ||
| Control | 50 | 18.20 ± 10.95 | ||
| Height (cm) | 6.746 | 0.034 | ||
| Cyanotic | 60 | 91.37 ± 26.05 | ||
| Acyanotic | 50 | 101.06 ± 25.13 | ||
| Control | 50 | 101.13 ± 23.93 | ||
| Microalbumin (mg) | 8.412 | 0.015 | ||
| Cyanotic | 60 | 14.51 ± 7.81 | ||
| Acyanotic | 50 | 10.20 ± 1.95 | ||
| Control | 50 | 10.31 ± 1.73 | ||
| BUN (mg/dL) | 0.046 | 0.977 | ||
| Cyanotic | 60 | 13.25 ± 4.04 | ||
| Acyanotic | 50 | 13.00 ± 3.25 | ||
| Control | 50 | 12.92 ± 2.80 | ||
| Creatinine (mg/dL) | 1.856 | 0.395 | ||
| Cyanotic | 60 | 0.56 ± 0.12 | ||
| Acyanotic | 50 | 0.53 ± 0.13 | ||
| Control | 50 | 0.53 ± 0.13 | ||
| Uric acid (mg/dL) | 54.042 | < 0.001 | ||
| Cyanotic | 60 | 5.26 ± 1.35 | ||
| Acyanotic | 50 | 3.48 ± 0.82 | ||
| Control | 50 | 3.62 ± 0.77 | ||
| Albumin/creatinine (mg/g) | 7.581 | 0.023 | ||
| Cyanotic | 60 | 26.59 ± 14.15 | ||
| Acyanotic | 50 | 20.57 ± 6.26 | ||
| Control | 50 | 20.47 ± 5.63 | ||
| GFR (mL/min/1.73 m2) | 19.874 | < 0.001 | ||
| Cyanotic | 60 | 85.15 ± 19.00 | ||
| Acyanotic | 50 | 102.61 ± 14.91 | ||
| Control | 50 | 101.64 ± 14.76 | ||
| Total | 160 | 95.76 ± 18.38 | ||
| Participants 5 - 15 years old | ||||
| Weight (kg) | 5.681 | 0.058 | ||
| Cyanotic | 21 | 22.32 ± 8.72 | ||
| Acyanotic | 21 | 27.71 ± 8.68 | ||
| Control | 18 | 28.42 ± 12.79 | ||
| Height (cm) | 1.27 | 0.53 | ||
| Cyanotic | 21 | 121.86 ± 15.66 | ||
| Acyanotic | 21 | 125.95 ± 16.13 | ||
| Control | 18 | 128.39 ± 15.19 | ||
| Microalbumin (mg) | ||||
| Cyanotic | 21 | 17.80 ± 9.46 | 7.739 | 0.021 |
| Acyanotic | 21 | 10.10 ± 2.39 | ||
| Control | 18 | 10.75 ± 1.74 | ||
| BUN (mg/dL) | 0.277 | 0.871 | ||
| Cyanotic | 21 | 14.81 ± 4.14 | ||
| Acyanotic | 21 | 15.33 ± 2.67 | ||
| Control | 18 | 15.44 ± 2.59 | ||
| Creatinine (mg/dL) | 2.742 | 0.254 | ||
| Cyanotic | 21 | 0.67 ± 0.11 | ||
| Acyanotic | 21 | 0.64 ± 0.09 | ||
| Control | 18 | 0.67 ± 0.09 | ||
| Uric acid (mg/dL) | 18.546 | < 0.001 | ||
| Cyanotic | 21 | 5.45 ± 1.46 | ||
| Acyanotic | 21 | 3.73 ± 0.76 | ||
| Control | 18 | 4.19 ± 0.68 | ||
| Albumin/creatinine (mg/g) | 7.55 | 0.023 | ||
| Cyanotic | 21 | 27.66 ± 17.16 | ||
| Acyanotic | 21 | 16.09 ± 4.32 | ||
| Control | 18 | 16.40 ± 3.90 | ||
| GFR (mL/min/1.73 m2) | 2.216 | 0.118 | ||
| Cyanotic | 21 | 101.46 ± 15.37 | ||
| Acyanotic | 21 | 109.46 ± 9.34 | ||
| Control | 18 | 106.17 ± 11.48 | ||
| Total | 60 | 105.67 ± 12.61 | ||
| Participants 1 - 5 years old | ||||
| Weight (kg) | 24.888 | < 0.001 | ||
| Cyanotic | 18 | 10.13 ± 1.56 | ||
| Acyanotic | 19 | 12.45 ± 2.39 | ||
| Control | 21 | 13.72 ± 1.32 | ||
| Height (cm) | 8.452 | 0.015 | ||
| Cyanotic | 18 | 85.44 ± 6.22 | ||
| Acyanotic | 19 | 89.53 ± 7.07 | ||
| Control | 21 | 92.11 ± 5.91 | ||
| Microalbumin (mg) | 5.615 | 0.06 | ||
| Cyanotic | 18 | 14.51 ± 8.03 | ||
| Acyanotic | 19 | 10.54 ± 1.72 | ||
| Control | 21 | 9.83 ± 1.59 | ||
| BUN (mg) | 1.301 | 0.522 | ||
| Cyanotic | 18 | 13.89 ± 4.21 | ||
| Acyanotic | 19 | 12.37 ± 2.24 | ||
| Control | 21 | 11.95 ± 1.53 | ||
| Creatinine (mg) | 9.229 | 0.01 | ||
| Cyanotic | 18 | 0.54 ± 0.08 | ||
| Acyanotic | 19 | 0.48 ± 0.07 | ||
| Control | 21 | 0.47 ± 0.05 | ||
| Uric acid (mg) | 30.197 | < 0.001 | ||
| Cyanotic | 18 | 5.72 ± 0.95 | ||
| Acyanotic | 19 | 3.31 ± 0.65 | ||
| Control | 21 | 3.29 ± 0.57 | ||
| Albumin/Creatinine (mg/g) | 1.225 | 0.542 | ||
| Cyanotic | 18 | 26.97 ± 16.00 | ||
| Acyanotic | 19 | 22.51 ± 5.81 | ||
| Control | 21 | 21.14 ± 4.82 | ||
| GFR (mL/min/1.73 m2) | 13.32 | < 0.001 | ||
| Cyanotic | 18 | 87.77 ± 12.40 | ||
| Acyanotic | 19 | 103.43 ± 14.27 | ||
| Control | 21 | 108.10 ± 11.33 | ||
| Total | 58 | 100.26 ± 15.18 | ||
| Participants less than 1 year old | ||||
| Weight (kg) | 26.672 | < 0.001 | ||
| Cyanotic | 21 | 6.50 ± 1.17 | ||
| Acyanotic | 10 | 8.15 ± 1.03 | ||
| Control | 11 | 10.01 ± 1.45 | ||
| Height (cm) | 15.745 | < 0.001 | ||
| Cyanotic | 21 | 65.95 ± 6.20 | ||
| Acyanotic | 10 | 70.70 ± 2.98 | ||
| Control | 11 | 73.73 ± 3.01 | ||
| Microalbumin (mg) | 1.353 | 0.508 | ||
| Cyanotic | 21 | 11.22 ± 3.57 | ||
| Acyanotic | 10 | 9.76 ± 1.30 | ||
| Control | 11 | 10.51 ± 1.90 | ||
| BUN (mg) | 3.334 | 0.189 | ||
| Cyanotic | 21 | 11.14 ± 2.89 | ||
| Acyanotic | 10 | 9.30 ± 1.70 | ||
| Control | 11 | 10.64 ± 1.75 | ||
| Creatinine (mg) | 10.24 | 0.006 | ||
| Cyanotic | 21 | 0.45 ± 0.06 | ||
| Acyanotic | 10 | 0.38 ± 0.05 | ||
| Control | 11 | 0.41 ± 0.04 | ||
| Uric acid (mg) | 12.976 | 0.002 | ||
| Cyanotic | 21 | 4.68 ± 1.36 | ||
| Acyanotic | 10 | 3.29 ± 1.13 | ||
| Control | 11 | 3.30 ± 0.74 | ||
| Albumin/creatinine (mg/g) | 4.637 | 0.098 | ||
| Cyanotic | 21 | 25.20 ± 8.68 | ||
| Acyanotic | 10 | 26.30 ± 3.66 | ||
| Control | 11 | 25.85 ± 4.50 | ||
| GFR (mL/min/1.73 m2) | 20.002 | < 0.001 | ||
| Cyanotic | 21 | 66.58 ± 7.07 | ||
| Acyanotic | 10 | 86.70 ± 14.75 | ||
| Control | 11 | 81.91 ± 5.79 | ||
| Total | 42 | 75.38 ± 12.75 |
Abbreviations: BUN, blood urea nitrogen; GFR, glomerular filtration rate.
For participants under 1 year of age, both height and weight were significantly reduced in children with CCHD compared to the other groups (P < 0.001). Creatinine levels were elevated in children with CCHD (0.45 ± 0.06 mg/dL) compared to those with aCCHD (0.38 ± 0.05 mg/dL) and controls (0.41 ± 0.04 mg/dL) (P = 0.006). No significant differences were observed in albumin/creatinine ratios between the groups (P = 0.098). The GFR was significantly lower in children with CCHD (66.58 ± 7.07 mL/min/1.73 m2) compared to both aCCHD (86.70 ± 14.75 mL/min/1.73 m2) and controls (81.91 ± 5.79 mL/min/1.73 m2) (P < 0.001).
Among participants aged 1 to 5 years, creatinine levels were significantly higher in children with CCHD (0.54 ± 0.08 mg/dL) compared to both aCCHD (0.48 ± 0.07 mg/dL) and controls (0.47 ± 0.05 mg/dL) (P = 0.001). Uric acid levels were also elevated in CCHD children (5.72 ± 0.95 mg/dL) compared to those with aCCHD (3.31 ± 0.65 mg/dL) and controls (3.29 ± 0.57 mg/dL) (P < 0.001). No significant differences were found in albumin/creatinine ratios between the groups (P = 0.542). The GFR was significantly lower in CCHD children (87.77 ± 12.40 mL/min/1.73 m2) compared to both aCCHD (103.43 ± 14.27 mL/min/1.73 m2) and controls (108.10 ± 11.33 mL/min/1.73 m2) (P < 0.001).
For participants aged 5 years and older, microalbuminuria was significantly higher in children with CCHD (17.80 ± 9.46 mg/mg) compared to those with aCCHD (10.10 ± 2.39 mg/mg) and controls (10.75 ± 1.74 mg/mg) (P = 0.021). Uric acid levels were also elevated in CCHD children (5.45 ± 1.46 mg/dL) compared to both aCCHD (3.73 ± 0.76 mg/dL) and controls (4.19 ± 0.68 mg/dL) (P < 0.001). No significant differences were found in creatinine, blood urea nitrogen (BUN), and GFR levels between the intervention groups. The albumin/creatinine ratio was significantly higher in children with CCHD (27.66 ± 17.16 mg/g) compared to those with aCCHD (16.09 ± 4.32 mg/g) and controls (16.40 ± 3.9 mg/g) (P = 0.023). Table 3 provides the pairwise comparisons for the significant variables related to weight, height, and renal involvement markers.
| Variables and Pairs | P-Value | |||
|---|---|---|---|---|
| All Participants | < 1 (y) | 1 - 5 (y) | 5 - 15 (y) | |
| Body weight (kg) | ||||
| Cyanotic-acyanotic | 0.001 | 0.003 | 0.001 | 0.027 |
| Cyanotic-control | < 0.001 | < 0.001 | < 0.001 | 0.044 |
| Acyanotic-control | 0.687 | 0.095 | 0.203 | 0.881 |
| Height (cm) | ||||
| Cyanotic-acyanotic | 0.032 | 0.030 | - | - |
| Cyanotic-control | 0.023 | < 0.001 | - | - |
| Acyanotic-control | 0.905 | 0.167 | - | - |
| Microalbumin (mg) | ||||
| Cyanotic-acyanotic | 0.012 | - | 0.059 | - |
| Cyanotic-control | 0.014 | - | 0.005 | - |
| Acyanotic-control | 0.958 | - | 0.332 | - |
| BUN (mg/dL) | ||||
| Cyanotic-acyanotic | - | - | - | - |
| Cyanotic-control | - | - | - | - |
| Acyanotic-control | - | - | - | - |
| Cr (mg/dL) | ||||
| Cyanotic-acyanotic | - | 0.003 | 0.028 | - |
| Cyanotic-control | - | 0.036 | 0.004 | - |
| Acyanotic-control | - | 0.413 | 0.501 | - |
| Uric acid (mg/dL) | ||||
| Cyanotic-acyanotic | < 0.001 | 0.005 | < 0.001 | < 0.001 |
| Cyanotic-control | < 0.001 | 0.002 | < 0.001 | < 0.001 |
| Acyanotic-control | 0.584 | 0.904 | 0.906 | 0.223 |
| Albumin/creatinine (mg/g) | ||||
| Cyanotic-acyanotic | 0.027 | - | - | - |
| Cyanotic-control | 0.014 | - | - | - |
| Acyanotic-control | 0.804 | - | - | - |
| GFR (mL/min/1.73 m2) | ||||
| Cyanotic-acyanotic | < 0.001 | < 0.001 | 0.001 | - |
| Cyanotic-control | < 0.001 | < 0.001 | < 0.001 | - |
| Acyanotic-control | 0.953 | 0.464 | 0.451 | - |
Abbreviations: BUN, blood urea nitrogen; GFR, glomerular filtration rate.
5. Discussion
The results of this study highlight significant differences in growth parameters and renal function markers between children with CCHD, aCCHD, and healthy controls. Children with CCHD exhibited significantly lower height and weight across all age groups, consistent with known growth delays in this population. Additionally, renal involvement was more pronounced in the CCHD group, as evidenced by elevated microalbuminuria, increased uric acid levels, and a higher albumin/creatinine ratio compared to the aCCHD and control groups. The reduced eGFR observed in CCHD children further underscores the impact of cyanotic heart defects on kidney function. These findings align with previous studies suggesting a higher risk of kidney dysfunction in CCHD patients, highlighting the need for early monitoring and intervention to prevent long-term renal complications in this vulnerable population.
The present study also found that microalbuminuria was significantly increased in children with CCHD, in all participants and in participants aged 1 - 5 years. Creatinine was significantly increased in CCHD only in the 5-year-old and younger age group. Uric acid levels were significantly increased in CCHD children in all age groups mentioned in the study. The microalbumin/creatinine ratio increased in CCHD children. Renal function in patients with CCHD has been less extensively studied, with relatively few studies focusing on renal function in infants and young children with CHD (9). Talolena et al. (6) observed that patients with CCHD had a higher risk of proteinuria compared to controls. Similarly, Zheng et al. (10) concluded that tubular injuries could occur in patients with CHD during infancy and early childhood, with these effects being most prominent in children with severe cyanosis. In our cohort, glomerular damage was noted in some children with cyanotic CHD. Gillesen et al. (11) found that the risk of kidney damage in patients with CHD was 6.4 times greater than in controls. Hamed et al. (2) evaluated 49 CHD patients and found a significant increase in microalbuminuria in children with CCHD but no notable differences in the microalbumin/creatinine ratio or GFR. In contrast, Isezuo et al. (12) conducted a study on 55 children, including 22 with aCCHD, 22 with CCHD, and 11 controls, and found the highest levels of microalbuminuria in children with CCHD, followed by those with aCCHD, while controls had the lowest levels. Another study reported that patients with CCHD had a higher incidence of renal failure and higher levels of abnormal biochemical markers compared to patients with aCCHD, although no difference was observed in the mean levels of microalbuminuria between the two groups (13).
Mohamed et al. (3) did not find a relationship between cyanosis and GFR levels, while Maleki et al. (14) found that the aCCHD group and severe CCHD group did not have significantly higher GFR compared to the mild CCHD group. In our study, regardless of age, GFR showed a downward trend in CCHD children compared to both the aCCHD and control groups. In the study by Mohamed et al. (3) GFR was not measured in children with aCCHD under one year, while 15.4% of the same age group with CCHD exhibited abnormal values significantly different from the control group. In children older than one year, 8.3% of aCCHD patients had abnormal GFR, while 25% of CCHD patients showed GFR values outside the normal range, significantly differing from both the acyanotic and control groups. Similar trends were observed for the albumin/creatinine ratio, with 7.7% of aCCHD children under one year having abnormal values, while 23.1% of age-matched CCHD patients had abnormal values. In subgroups older than one year, 16.7% of aCCHD children had abnormal values, while 33.3% of CCHD patients showed U Alb/Cr ratios outside the normal range. Both acyanotic and cyanotic groups showed no significant difference compared to controls.
Fang et al. (5) conducted a study on 359 patients with CHD, of whom 46.5% had CKD, including 18 patients with clinical CKD and 149 with non-clinical CKD. The study found that patients with clinical CKD were significantly older at enrollment than those with non-clinical CKD. The authors also demonstrated that the incidence of kidney damage increased with age in children with CHD, which contrasts with the results of the present study, where microalbuminuria, creatinine, and albumin/creatinine were not significantly different in children with CHD aged 5 - 15 years. A similar study of 94 children with CCHD identified a case of microalbuminuria in a one-month-old child (15). Amoozgar et al. (16) confirmed that children with CCHD are at greater risk for kidney damage, which is more closely related to the duration of cyanosis, a proxy for age. Fang et al. (5) also found that age is an independent risk factor for kidney injury in patients with CHD. In children with CHD, CKD was found in 2 - 3% during childhood, increasing to 7 - 14% in adolescence and young adulthood. This increase in kidney disease is thought to be related to the duration of cyanosis and polycythemia, with most reports coming from adults who survived in developed countries with optimal cardiac care (17).
The findings in the current study regarding microalbuminuria, albumin/creatinine ratio, and GFR align with these trends. As the kidneys age, they naturally lose some nephrons, and the remaining nephrons may not function as efficiently. In the case of prolonged cyanosis, this can have a devastating impact on renal function, leading to both glomerular and tubular dysfunction. Reduced cardiac output from chronic heart failure further exacerbates this, causing decreased renal perfusion over time (15). Recent studies have highlighted cardiac involvement as a major risk factor for kidney disease. When the heart’s pumping efficiency declines, it leads to congestion, which raises pressure in the vena cava, and consequently, blood flow to the kidneys is impaired. The highest risk of kidney damage (CKD) is seen in the most complex CHD cases, particularly severe non-conotruncal and conotruncal lesions. This is consistent with previous studies, which suggest that anatomical complexity and cyanosis are strongly associated with renal dysfunction. Patients with complex lesions often require multiple cardiac surgeries, increasing their risk for acute kidney injury (AKI) and, consequently, CKD (18-20).
The findings of the present study align with those of previous research, with slight differences explained by variations in patient age, methodologies, and the severity of cyanosis. This study reveals that children with aCCHD have a relatively lower absolute risk of kidney disease compared to those with CCHD. To assess the absolute risk of kidney disease in CHD patients, future studies should include clinical evaluations to identify potential risk factors such as comorbidities, lifestyle, or coronary artery disease, as well as accurate GFR measurements, which are not available through current registries. Investigating the nephrotoxicity of medical therapies, with or without congenital heart defects, would also be valuable. Gathering more detailed patient data would provide insights into how and why kidney disease develops in CHD patients and help identify those at high risk. The results of such studies could lead to better screening and monitoring guidelines for renal function in CHD patients, facilitating early detection of kidney failure, slowing disease progression, and identifying preventable risk factors for CKD.
5.1. Conclusions
This study demonstrates significant renal dysfunction in children with CHD, particularly in those with CCHD. Children with CCHD exhibited reduced growth parameters, including lower height and weight, along with elevated renal markers such as microalbuminuria, uric acid levels, and albumin/creatinine ratios, compared to both aCCHD children and healthy controls. Furthermore, the eGFR was significantly lower in the CCHD group, suggesting impaired kidney function. These findings emphasize the need for early renal assessment and continuous monitoring in children with CCHD, as renal involvement is prevalent and may lead to long-term health complications. The results support the development of targeted prevention strategies and the implementation of more comprehensive renal follow-up protocols in this high-risk population.
5.2. Study Limitations
There were several limitations to our study. Notably, there were no data from renal histology to confirm CN diagnoses, which would demonstrate the effect of CHD on nephron injury. Further prospective cohort studies focusing on albuminuria as a predictor of the development of CN, as well as randomized controlled studies to clarify the benefit of angiotensin-converting enzyme inhibitors (ACEIs) in nephropathy diagnoses, will be required.