1. Background
The 2019 coronavirus pandemic (COVID-19) has had a profound global impact, affecting 212 countries and resulting in a significant mortality rate (1). Individuals with underlying cardiovascular disease are at increased risk of severe disease and death, with a mortality rate of 10.5% (2). While COVID-19 is primarily known for its respiratory symptoms, ranging from mild upper respiratory symptoms to acute respiratory distress syndrome (ARDS), some patients initially present with cardiovascular symptoms such as chest discomfort, palpitations, and dyspnea (3, 4). In these cases, it can be challenging for clinicians to determine whether the symptoms indicate SARS-CoV-2 cardiac involvement or stem from an underlying pathological cardiac condition. Several cases of myocardial injury caused by COVID-19 have been observed, including acute myocardial injury, myocarditis, cardiac arrest, heart failure, and pulmonary hypertension (5, 6). In COVID-19, cardiovascular manifestations can result from primary or secondary cardiac involvement or even worsening of pre-existing cardiovascular disease (CVD). Echocardiography may provide information about the mechanism, type, and extent of effects (7). Several studies have provided information on different types of cardiac involvement, with varying results on the frequency of right ventricular (RV) or left ventricular (LV) involvement, possibly due to the diverse populations analyzed. Some studies performed echocardiograms on every person with COVID-19, while others included only those with a clear indication for echocardiography, such as chest pain, elevated troponin, or hemodynamic instability (8-10). Additionally, disease severity varied between studies, complicating data interpretation. In cases with a previous history of CVDs, echocardiography can support diagnosis and detect signs suggestive of acute myocardial infarction, new onset or worsening congestive heart failure, pericardial effusion or tamponade, and RV overload due to pulmonary or cardiopulmonary embolism (11, 12). Echocardiography is a valuable diagnostic tool for evaluating myocardial structure and function due to its availability and cost-effectiveness (13). As recommended by the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE), the informed use of transthoracic echocardiography (TTE) in hospital settings using a focused and safe approach can reduce the risk of heart damage and provide timely diagnosis and treatment (14, 15).
2. Objectives
To better understand the effect of changes caused by this viral infection on the formation or development of cardiac complications and to explore their potential as predictors of cardiac risk, this study aimed to investigate the late findings of echocardiography in patients with COVID-19, with a particular focus on RV assessment.
3. Methods
3.1. Participants
This descriptive and analytical cross-sectional study enrolled patients diagnosed with COVID-19 at Modarres Hospital from March 2020 to February 2022. A non-random, consecutive sampling strategy was employed to select 90 participants based on predefined inclusion and exclusion criteria. The sample size was determined as 90 cases using G-power software, considering a study power of 95% and an α error of 5%. The inclusion criteria for study participants included the absence of a history of heart bypass surgery, congenital heart disease, recent myocardial infarction (less than 6 weeks prior), history of cancer, and the absence of restrictive and hypertrophic cardiomyopathy, pericardial diseases, or valve diseases. This study followed the STROBE guidelines and was conducted in line with the principles of the Declaration of Helsinki and according to the accepted regulations of the Shahid Beheshti University of Medical Sciences ethical committee (IR.SBMU.RETECH.REC.1402.533). Informed consent was obtained from all participants.
3.2. Classification and Evaluation of Patients
Following the selection of eligible patients, their echocardiographic findings were recorded based on clinical files. Written informed consent, including an explanation of the purpose and procedure of the research during the two-year follow-up, was obtained from all participants. A standardized transthoracic echocardiographic (TTE) assessment was performed using a 2D echocardiographic machine (Philips USA, EPIC Q CV x 3D, Probe X51). The examination included measurements of left and RV function, chamber sizes, wall thickness, valvular function, and pulmonary pressures, following the guidelines of the American Society of Echocardiography (ASE). Standard parasternal, apical, and subcostal views were obtained, and Doppler imaging was used to assess blood flow velocities and diastolic function. All measurements were averaged over three cardiac cycles to ensure accuracy and reproducibility. The echocardiographic parameters evaluated included left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), interventricular septal diameter (IVSD), E/A ratio, E wave velocity, Left Atrial Volume Index (LAVI), left ventricular global longitudinal strain (LVGLS), right ventricular end-diastolic diameter (RVEDD), fractional area change (FAC), S wave velocity, tricuspid annular plane systolic excursion (TAPSE), tricuspid regurgitation velocity (TR velocity), right ventricular global longitudinal strain (RVGLS), and systolic pulmonary artery pressure (SPAP). Changes in these factors were then assessed over the study period.
3.3. Statistical Analysis
Data were analyzed using SPSS version 22 software. Descriptive statistics reported quantitative variables as means and standard deviations, while qualitative variables were presented as counts and percentages. The chi-square test was employed to compare qualitative variables between groups, and the paired sample t-test was utilized for comparisons of quantitative variables. A significance level of P ≤ 0.05 was set for all statistical tests.
4. Results
4.1. Baseline Characteristics
As shown in Table 1, regarding qualitative characteristics, 52.2% of patients were male, 21.1% had a history of diabetes mellitus, 26.7% were diagnosed with hypertension, and 1.1% reported a history of smoking. Notably, none of the patients had a family history of heart disease. Additionally, 12.2% of the cohort had hyperlipidemia and ischemic heart disease (IHD). In terms of quantitative characteristics, the mean age of the patients was 34.5 years (± 13.7), with a range from 17 to 88 years. The average Body Mass Index (BMI) was 31.8 kg/m2 (± 8.0), with values ranging from 25.1 to 46.6 kg/m2. The patients had an average weight of 78.47 kg (± 17.34) and a mean height of 167.24 cm (± 19.15), with heights ranging from 151 to 188 cm. Systolic blood pressure (BP) averaged 117.83 mmHg (± 12.78), with readings spanning from 100 to 180 mmHg, while the mean heart rate was 74.42 beats per minute (± 6.74), ranging from 60 to 92 bpm.
| Parameters | Values |
|---|---|
| Qualitative variables | |
| Gender (male) | 47 (52.2) |
| Diabetes mellitus (yes) | 19 (21.1) |
| Hypertension (yes) | 24 (26.7) |
| Smoking (yes) | 1 (1.1) |
| Family history (yes) | 0 (0) |
| Hyperlipidemia (yes) | 11 (12.2) |
| Ischemic heart disease (yes) | 11 (12.2) |
| Quantitative variables | |
| Age (y) | 34.5 ± 13.7 (17 - 88) |
| BMI (kg/m2) | 31.8 ± 8.0 (25.1 - 46.6) |
| Weight (kg) | 78.47 ± 17.34 (50 - 157) |
| Height (cm) | 167.24 ± 19.15 (151 - 188) |
| Systolic BP (mmHg) | 117.83 ± 12.78 (100 - 180) |
| Heart rate (bpm) | 74.42 ± 6.74 (60 - 92) |
Demographic Characteristics of COVID-19 Patients a
4.2. Comparison of Echocardiographic Variables
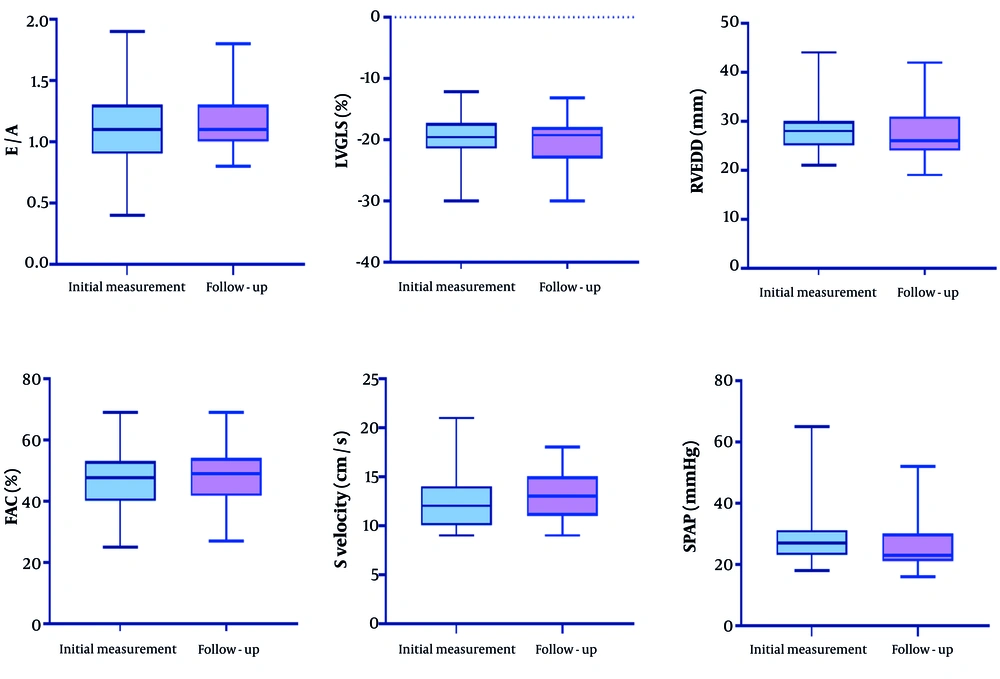
Using a paired sample t-test, we analyzed the echocardiographic variables of patients and found that the differences in E/A, LVGLS, RVEDD, FAC, S-velocity, RVGLS, and pulmonary artery pressure (PAP) were statistically significant. Specifically, the E/A ratio increased from 1.09 ± 0.27 at the initial measurement to 1.15 ± 0.23 during follow-up (P = 0.01), indicating a significant improvement. Additionally, LVGLS showed a significant change, moving from -19.24 ± 5.18% to -20.38 ± 3.59% (P = 0.005). The RVEDD decreased significantly from 28.07 ± 4.31 mm to 27.32 ± 4.73 mm (P = 0.003), while fractional area change (FAC) improved from 46.6 ± 9.16% to 47.54 ± 7.9% (P = 0.02). S-velocity also demonstrated notable improvement, increasing from 12.16 ± 2.32 cm/s to 13.02 ± 2.2 cm/s (P = 0.001). The SPAP measurements indicated a significant decrease from 28.48 ± 7.56 mm Hg to 26.0 ± 6.7 mm Hg (P = 0.001). The RVGLS showed a significant change, from -22.47 ± 7.22% to -23.04 ± 3.23% (P = 0.001). The detailed results are summarized in Table 2 and illustrated in Figure 1.
| Parameters | Initial Values | Follow-up Values | P-Value |
|---|---|---|---|
| LVEF (%) | 60.66 ± 7.51 | 59.79 ± 9.33 | 0.31 |
| LVEDD (mm) | 45.05 ± 4.7 | 44.95 ± 4.56 | 0.74 |
| LVESD (mm) | 29.86 ± 5.28 | 30.02 ± 5.25 | 0.59 |
| IVSD (cm) | 9.96 ± 1.65 | 9.71 ± 1.95 | 0.06 |
| E/A | 1.09 ± 0.27 | 1.15 ± 0.23 | 0.01 |
| E (m/s) | 8.07 ± 2.19 | 7.83 ± 2.28 | 0.11 |
| LAVi (mL/m2) | 29.96 ± 5.8 | 29.13 ± 4.99 | 0.64 |
| LVGLS (%) | -19.24 ± 5.18 | -20.38 ± 3.59 | 0.005 |
| RVEDD (mm) | 28.07 ± 4.31 | 27.32 ± 4.73 | 0.003 |
| FAC (%) | 46.6 ± 9.16 | 47.54 ± 7.9 | 0.02 |
| S velocity (cm/s) | 12.16 ± 2.32 | 13.02 ± 2.2 | 0.001 |
| TAPSE (mm) | 21.03 ± 3.04 | 21.24 ± 2.64 | 0.41 |
| TR velocity (m/s) | 2.36 ± 0.38 | 2.9 ± 5.05 | 0.3 |
| SPAP (mmHg) | 28.48 ± 7.56 | 26.0 ± 6.7 | 0.001 |
| RV GLS (%) | -22.47 ± 7.22 | -23.04 ± 3.23 | 0.001 |
Comparison of Echocardiography Parameters a
5. Discussion
Myocardial involvement has been recognized as an early manifestation of COVID-19 infection relative to other members of the coronavirus family, with studies indicating that up to 30% of COVID-19 patients may experience irreversible myocardial involvement (16-20). In our study, initial echocardiography findings showed that while LVGLS was slightly decreased and SPAP was slightly increased, other parameters remained within normal ranges. This suggested that 2D speckle-tracking echocardiography (2D-STE) could be more beneficial than traditional echocardiographic parameters for evaluating the patients. The comparative analysis of echocardiographic findings between the initial examination and follow-up conducted two years later revealed significant improvements in E/A ratio, LVGLS, RVGLS, RVEDD, fractional area change (FAC), S-velocity, and SPAP. These changes likely indicate recovery from the acute myocardial injury sustained during the initial illness. Our findings regarding LV and RV volume and function during hospitalization for COVID-19 were consistent with other echocardiographic studies, which have demonstrated that absolute values of LVEF, E/e' ratio, TAPSE, and RV S' typically remain within normal limits, while GLS decreases during hospitalization (10, 21, 22). This pattern of "reversion to the mean" likely resulted from the resolution of the physiological stress response in acute patients. Our results provide valuable insights into the prevalence of cardiovascular sequelae following SARS-CoV-2 infection among patients who required hospitalization and survived for two years post-discharge. Previous studies have indicated that LVGLS was more sensitive in detecting subtle changes in LV function compared to LVEF (23). This finding aligned with our cohort, where LVGLS exhibited a significant decrease over time, despite initially normal LVEF. It has been hypothesized that LVEF may be affected by the presence of myocardial dysfunction but is also load-dependent (e.g., influenced by hypovolemia), while GLS may better reflect myocardial dysfunction and various aspects of the cardiac response to sepsis (24, 25). A study by Lassen et al. found that echocardiographic measures of RV function and cardiac biomarkers improved after resolution of COVID-19 infection, while LVEF showed a decrease post-recovery without a significant difference from LVEF in acute COVID-19 patients. They reported that GLS remained unchanged after recovery, and recovered COVID-19 patients exhibited lower GLS, TAPSE, and RVLS than matched controls, while still within clinically acceptable normal ranges (26). Moreover, a recent study by Moody et al. investigated adverse ventricular remodeling in 79 survivors of COVID-19, finding that 29% exhibited persistent adverse remodeling (27). However, their study had a significant selection bias since it included only patients who underwent echocardiography due to clinical complications during hospitalization. This selection likely accounts for the higher rates of cardiac complications observed in their population. Conversely, Catena et al. conducted echocardiographic examinations in 64 patients previously hospitalized for COVID-19, approximately 41 days after discharge, concluding that there were no abnormalities or evidence of persistent cardiac dysfunction (28). Unfortunately, they did not have acute infection data for comparison and lacked a non-COVID-19 control group. Furthermore, their study did not employ STE analysis to detect more subtle myocardial changes.
In agreement with our findings, several studies have emphasized that despite a slight decrease in LVEF, TAPSE, RVLS, and GLS, all metrics remained within normal ranges and clinically acceptable values. The clinically significant decrease in cardiac function observed did not warrant any clinical intervention. However, it is important to note that subclinical changes in myocardial function have been associated with poorer long-term prognoses in various studies, and the evidence for intervention based on these subclinical changes remains lacking (26, 29). Our results also align with findings from smaller studies using cardiac magnetic resonance imaging (cMRI) to assess diffuse cardiac inflammatory involvement in COVID-19 (30-32), particularly those reported by Puntmann et al. (33). Unlike these prior studies, our research demonstrated changes in cardiac function both during the acute infection phase and after recovery. Given that the lungs are the primary target organ for SARS-CoV-2 and considering the high incidence of acute respiratory distress syndrome in critically ill patients, it is hypothesized that the RV may be particularly vulnerable to dysfunction following COVID-19 infection. Prior studies have established RV failure as a consequence of acute lung injury and acute respiratory distress syndrome, as the RV is sensitive to changes in pulmonary vascular resistance. In our study, improvement in RVGLS suggested enhanced lung function from baseline to follow-up. Although echocardiography and cMRI have demonstrated RV dysfunction in COVID-19 survivors (34, 35), RV strain calculations are recommended as a clinical measure to evaluate RV function in suspected dysfunction scenarios (36). While our results showed that traditional RV morphological and functional parameters remained within normal ranges, RV strain in COVID-19 patients increased significantly during follow-up. We anticipated the most significant changes in functional parameters would occur between hospitalization and follow-up, which was indeed observed for RVGLS and SPAP. The change in RV S over time is clinically relevant, as the RV S values at follow-up remained normal for all patients. The most considerable difference was noted in GLS, which during hospitalization was associated with elevated inflammatory markers and hypoxia, suggesting that decreased GLS could be secondary to systemic inflammation (22). Although we did not evaluate inflammatory markers at follow-up, their normalization could explain the trend of improved GLS compared to hospitalization. In a study on a large cohort of 749 SARS-CoV-2 patients, it was observed that RV systolic dysfunction was more common than LV systolic dysfunction. They showed that a large proportion of patients had reduced RV function (8). While they focused on the performance of TTE in this disease, our study was designed to survey the improvement of parameters in a 2-month follow-up. Our data showed a notable recovery in several measures two years post-hospitalization. This hypothesis was supported by a global survey which showed that imaging changed management in one-third of COVID-19 patients (9). Our findings implied that, following the resolution of pneumonia caused by SARS-CoV-2, acute RV dysfunction, which may stem from pulmonary pathology-induced increases in RV afterload, may be improved. However, diffuse inflammation that could potentially affect LV function could still linger during the early recovery phase. The exact mechanisms leading to reduced LV myocardial function remain unclear. This reduction may result from direct viral damage to the heart, secondary systemic inflammation, or both (26).
The strengths of our prospective study design include repeated echocardiographic assessments during both acute infection and recovery phases. Unlike many retrospective studies that select patients based on perceived clinical deterioration, which may bias the prevalence of cardiac dysfunction, our approach allows for a more accurate representation of the cardiac status of unselected consecutive patients. However, our study has limitations. First, the sample size was relatively small, necessitating caution when interpreting sub-analyses. Second, we did not have access to inflammatory biomarkers at follow-up. Notably, patients who died, likely exhibiting the most severe heart dysfunction, were not included in our analysis. Nonetheless, this study provides valuable insights into the role of echocardiography in assessing patients with acute SARS-CoV-2 infection and highlights differences in baseline LV and RV function. Future research should involve comprehensive evaluations of cardiac function concerning echocardiographic changes in larger, multicenter cohorts. Focused assessments of non-invasive, detailed echocardiographic parameters in COVID-19 patients across all stages of the disease are recommended. Additionally, while we considered significant demographic aspects in our analysis, other determining factors, such as medication use, were not accounted for.
5.1. Conclusions
According to our findings, patients recovering from COVID-19 exhibit significant long-term improvements in cardiac parameters. While these improvements were generally within normal ranges, LVGLS and SPAP showed minimal disturbances in cardiac function during the initial examinations. This suggested their potential utility as non-invasive tools for patient assessment. Thus, this study provides valuable insights into long-term echocardiographic changes in COVID-19 patients, indicating potential reversibility of myocardial involvement.