1. Background
Tetralogy of Fallot (ToF) is the most common form of cyanotic congenital heart disease, occurring in approximately 3.26 cases per 10,000 live births and representing up to 10% of all congenital heart defects. It is characterized by four key anatomical features: Obstruction of the blood flow pathway to the lungs (infundibular pulmonary stenosis), ventricular septal defect (VSD), an overriding aortic root, and right ventricular hypertrophy. While cyanosis, the hallmark of ToF, may not be apparent during the neonatal period, it typically manifests as a right-to-left shunt develops. Additional symptoms include heart murmur, hyper-cyanotic episodes (known as "tet spells"), tachypnea, feeding-related diaphoresis, feeding intolerance, and poor weight gain. The severity of symptoms depends on the degree of obstruction to pulmonary blood flow (1).
The first surgical repair of ToF was reported by Lillehei et al. in 1955 (2). Since then, optimal surgical management has remained a topic of discussion. While many centers prefer performing a complete repair in a single stage, others favor a two-stage method that begins with shunt insertion and is followed by complete surgical correction at a later time (3). One of the most critical factors in determining surgical strategy and predicting outcomes such as mortality rate or the need for reoperation (4, 5) is the growth and size of the pulmonary arteries (4). Smaller pulmonary arteries have been associated with a higher risk of adverse surgical outcomes (5). Consequently, several methods and indices have been developed to quantify pulmonary artery dimensions (6, 7).
The McGoon ratio is one such index, measuring the sum of the diameter of the largest pulmonary artery branches at the pre-branching point relative to the diameter of the descending aorta at the diaphragm level. It can be obtained via cardiac catheterization (8). The Nakata Index, introduced by Nakata et al., represents the sum of the cross-sectional areas (CSAs) of the pulmonary arteries divided by body surface area (BSA) (6, 9). This Index can be assessed using computed tomography (CT) (8), echocardiography (10), or cardiac catheterization (6).
Although cardiac catheterization remains the gold standard for hemodynamic evaluation, it is an invasive procedure requiring imaging from various angles using contrast agents (11). For this reason, researchers are exploring alternatives, like echocardiography, which is the most common, noninvasive, and accessible imaging method for assessing congenital heart diseases (12). Recent advancements in echocardiography have significantly enhanced its utility as a primary diagnostic tool in the management of patients with ToF. Echocardiography, particularly advanced techniques such as 3D echocardiography, strain imaging, and intraoperative echocardiography, has improved the ability to assess cardiac anatomy and function in real-time (13).
For instance, Hamza et al. investigated the reliability of echocardiography in assessing the status of pulmonary blood flow in children with ToF, comparing it with measurements obtained via catheterization and during surgery. They concluded that it may be feasible to proceed with surgery — whether shunt creation or complete repair — based on echocardiography alone, given the risks, costs, and long waitlists associated with catheterization (14). However, the accuracy of echocardiography results is highly dependent on the skill of the operator and can also be affected by factors such as air or bone interference (11).
2. Objectives
Despite findings supporting the use of echocardiography as an alternative to cardiac catheterization, the present study aims to compare the diagnostic accuracy of echocardiography and catheterization, using Nakata and McGoon indices to guide surgical decision-making in patients with ToF.
3. Methods
3.1. Study Design
This was an analytical epidemiological study conducted to assess the diagnostic value of echocardiographic and angiographic (catheter-based) measurements in patients with ToF.
3.2. Study Population and Setting
The study was conducted at Imam Reza Hospital in Mashhad, Iran. Participants included patients diagnosed with ToF who were referred for cardiac evaluation and treatment.
3.3. Sampling Method and Sample Size
Participants were selected through a purposive (non-random) sampling method. The sample size was calculated based on an expected sensitivity of 60% for echocardiography, as referenced in prior studies by Rao et al. (15) and Raval et al. (16). Using a significance level (α) of 0.05 and a margin of error (d) of 0.12, the minimum required sample size was determined to be 65. Ultimately, 120 patients were enrolled.
3.4. Inclusion Criteria
- Patients with a confirmed diagnosis of ToF who presented to Imam Reza Hospital, Mashhad, Iran, to determine the treatment method.
3.5. Exclusion Criteria
- Refusal of participation by the patient or legal guardians
- Presence of additional congenital anomalies besides ToF
3.6. Data Collection Procedures
3.6.1. Cardiac Catheterization Assessment
Cardiac catheterization was performed first. Pulmonary artery branch diameters were measured in the anteroposterior (AP)/cranial view, while the descending aorta was measured in the AP view.
3.6.2. Echocardiographic Assessment
Echocardiography was performed within one week of cardiac catheterization using a Philips EPIQ 7c ultrasound system (Philips Healthcare, Bothell, WA, USA). Measurements of the right and left pulmonary arteries and the descending aorta (at the diaphragm level) were obtained in both parasternal and suprasternal views. All procedures were conducted by two experienced pediatric cardiologists.
3.7. Index Definitions and Calculations
Based on the measurements obtained, the Nakata and McGoon indices were calculated:
- McGoon Ratio = (Diameter of RPA + LPA)/Diameter of the descending aorta
- Nakata Index = (CSA of RPA + LPA)/BSA
All measurements from cardiac catheterization were analyzed using SPSS software (version 16, Chicago, IL, USA, 2007). Z-scores were applied where appropriate.
3.7.1. Cut-off Values
- McGoon Ratio (17):
- ≥ 2.1: Normal
- 1.2 - 2.1: Adequate for ToF repair
- ≤ 0.8: Inadequate for complete repair
- Nakata Index: As studies have suggested different cut-off values (6, 8, 17-19), this study adopted the following thresholds:
- > 180 mm2/m2: Normal
- ≤ 180 mm2/m2: Abnormal
3.8. Statistical Analysis
Although 120 patients were initially enrolled in the study, certain parameters could not be measured in all cases due to existing limitations. For example, complete cardiac catheterization data were only available for 63 individuals. Nevertheless, the available data were analyzed and reported accordingly. Statistical analysis included both descriptive and inferential methods:
3.8.1. Descriptive Statistics
Frequency, mean, median, and interquartile range were reported. Due to non-normal data distribution, medians were emphasized.
3.8.2. Analytical Statistics
- The Wilcoxon signed-rank test was used for comparing paired non-parametric measurements (echocardiography vs. catheterization).
- Spearman’s rank correlation was used to evaluate the strength of association between imaging modalities.
- A P-value < 0.05 was considered statistically significant.
4. Results
A total of 120 patients with ToF were enrolled in the study. Of these, 63 (52.5%) were male and 57 (47.5%) were female. Age of patients ranged from 10 months to 24 years, with a mean age of 3.41 ± 4.39 years. Body weight ranged from 2.1 to 65.0 kg, with a mean of 12.00 ± 10.72 kg. Demographic data are summarized in Table 1.
| Descriptive Statistics | Valid (No.) | Range | Mean ± SD |
|---|---|---|---|
| Body weight (kg) | 93 | 2.10 - 65.00 | 12.00 ± 10.72 |
| Age (y) | 116 | 0.10 - 24.00 | 3.41 ± 4.39 |
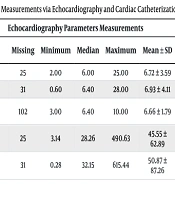
The diameters of the right and left pulmonary arteries and the diameter of the aorta were measured using both echocardiography and cardiac catheterization. Measurements of the left and right cross-sectional areas (CSA-L and CSA-R) were also recorded. The results are presented in Table 2.
| Parameters | Echocardiography Parameters Measurements | Cardiac Catheterization Parameters Measurements | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Valid (No.) | Missing | Minimum | Median | Maximum | Mean ± SD | Valid (No.) | Missing | Minimum | Median | Maximum | Mean ± SD | |
| LPA size (mm) | 95 | 25 | 2.00 | 6.00 | 25.00 | 6.72 ± 3.59 | 63 | 57 | 2.20 | 9.20 | 24.00 | 10.06 ± 5.18 |
| RPA size (mm) | 89 | 31 | 0.60 | 6.40 | 28.00 | 6.93 ± 4.11 | 63 | 57 | 1.70 | 8.80 | 19.00 | 9.66 ± 4.12 |
| Descending aorta size (mm) | 18 | 102 | 3.00 | 6.40 | 10.00 | 6.66 ± 1.79 | 63 | 57 | 5.60 | 8.09 | 24.00 | 9.10 ± 3.06 |
| CSA-L | 95 | 25 | 3.14 | 28.26 | 490.63 | 45.55 ± 62.89 | 63 | 57 | 3.80 | 66.44 | 452.16 | 100.32 ± 106.52 |
| CSA-R | 89 | 31 | 0.28 | 32.15 | 615.44 | 50.87 ± 87.26 | 63 | 57 | 2.27 | 60.79 | 283.39 | 86.50 ± 68.70 |
Abbreviations: LPA, left pulmonary artery; RPA, right pulmonary artery; CSA-L, left cross-sectional area; CSA-R, right cross-sectional area.
The McGoon ratio and Nakata Index were calculated using data from both imaging modalities.
| Measurements Methods | McGoon Ratio Measurements | Nakata Index Measurements | ||
|---|---|---|---|---|
| Range | Mean ± SD | Range | Mean ± SD | |
| Echocardiography | 0.71 - 2.95 | 1.67 ± 0.51 | 7.95 - 1106.07 | 96.70 ± 145.89 |
| Catheterization | 0.42 - 4.20 | 2.19 ± 0.72 | 12.35 - 735.55 | 186.82 ± 156.36 |
In the next step, patients were classified into normal and abnormal groups based on defined cut-off values for both indices. The distribution is shown in Table 4.
| Index | McGoon Index in Cardiac Catheterization | McGoon Index in Echocardiography | Nakata index in Cardiac Catheterization | Nakata Index in Echocardiography |
|---|---|---|---|---|
| Normal | 31 (49.2) | 2 (11.1) | 24 (38.1) | 8 (9.3) |
| Abnormal | 32 (50.8) | 16 (88.9) | 39 (61.9) | 78 (90.7) |
| Total | 63 (100) | 18 (100) | 63 (100) | 86 (100) |
A Wilcoxon signed-rank test revealed a statistically significant difference between values obtained via echocardiography and cardiac catheterization (P = 0.009). Spearman’s rank correlation was used to assess agreement between methods. Results are summarized in Table 5.
Abbreviations: LPA, left pulmonary artery; RPA, right pulmonary artery; CSA-L, left cross-sectional area; CSA-R, right cross-sectional area.
a Correlation at P < 0.05 is considered statistically significant.
A statistically significant positive correlation was observed for RPA (ρ = 0.418; P = 0.004), descending aorta (ρ = 0.652; P = 0.041), and CSA-R (ρ = 0.418; P = 0.004). No significant correlation was found for the McGoon ratio (ρ = 0.067; P = 0.855) or for LPA and CSA-L measurements.
5. Discussion
Echocardiography and cardiac catheterization have been used to evaluate congenital heart diseases (20). Echocardiography is often recognized as the primary diagnostic modality due to its widespread availability, ease of use (12), and ability to visualize cardiac structures and estimate cardiac hemodynamics (21). This study aimed to compare the diagnostic accuracy of echocardiography versus cardiac catheterization by assessing two established indices, the Nakata Index and the McGoon ratio, for surgical decision-making in patients with ToF.
Our findings demonstrated that echocardiographic measurements of the right and left pulmonary arteries and the descending aorta were consistently lower than those obtained through cardiac catheterization (Table 2). Kumar et al. evaluated the reliability and correlation between findings of echocardiography and cardiac catheterization, considered a mandatory test for planning surgical or transcatheter interventions to assess pulmonary vascular dimensions, in 54 patients aged 3 - 34 years with ToF. They found that pulmonary vascular parameters assessed by echocardiography were significantly lower than those measured by cardiac catheterization, reaffirming the greater accuracy of catheter-based evaluation in this setting. However, due to its accessibility, non-invasive nature, and acceptable diagnostic correlation with cardiac catheterization findings, they recommended echocardiography as a screening tool, emphasizing that definitive surgical decision-making should rely on scientific evidence rather than expert opinion alone (22).
Similarly, Hamza et al. investigated two-dimensional echocardiography for assessing pulmonary blood flow in 18 children with ToF and found that echocardiographic measurements of the RPA and LPA diameters were lower than those obtained through cardiac catheterization (14), further supporting our results in Table 2. The relatively smaller values obtained by echocardiography may be influenced by various factors, including operator experience, limited acoustic windows, the inherent hypoplastic tendency of the pulmonary vessels in ToF, and anatomical challenges such as angulation of the LPA (22).
In our study, the McGoon Index values and the proportion of patients classified as "normal" (McGoon ratio > 2.1) were higher when calculated from catheterization data than from echocardiographic measurements, consistent with the generally larger diameters observed on cardiac catheterization. Also, analysis of the Nakata Index revealed that cardiac catheterization identified 24 out of 63 patients (38.1%) as having normal pulmonary artery size. In comparison, echocardiography identified only 8 out of 86 patients (9.3%) in the normal range (Table 4). This discrepancy may partly result from the mathematical nature of the Nakata Index, which is calculated from the cross-sectional area of pulmonary arteries based on squared vessel diameters. Consequently, small underestimations in diameter, more likely in echocardiography due to its acoustic and technical limitations, can lead to disproportionately large reductions in the calculated index.
While this is a known mathematical principle, our findings align with those of Kumar et al., who similarly observed that Nakata Index values derived from echocardiography were significantly lower than those from catheterization (22). Our findings revealed a statistically significant correlation between echocardiographic and cardiac catheterization results (P = 0.009). This result suggests that echocardiography alone may not provide accurate measurements of pulmonary artery size or reliable calculations of the Nakata and McGoon indices.
According to Kumar et al.’s study, pulmonary vascular indices differed between echocardiography and cardiac catheterization but showed a statistically significant correlation. Although the data confirmed the superiority of cardiac catheterization over echocardiography in ToF, the strong diagnostic correlation and benefits of echocardiography as a cheap, accessible, and non-invasive method support its application as a screening tool within the evaluated population. This approach supports surgical decision-making in ToF based on scientific evidence rather than solely on expert opinion (22). This is consistent with the correlation between echocardiographic and catheterization measurements of the present study, which showed that while significant correlations were observed for parameters such as RPA, CSA-R, and the descending aorta, others, including LPA and the McGoon ratio, did not show statistically meaningful agreement between the two imaging modalities. Therefore, echocardiography may serve as a useful screening tool, but cardiac catheterization remains more accurate, particularly when precise vascular sizing is required for surgical decision-making.
However, some other studies have also reported a high diagnostic correlation between echocardiography and angiocardiography results, recommending that surgical decisions in uncomplicated ToF cases can be based on echocardiography alone, without the need for cardiac catheterization (14, 21, 23). Echocardiography has limitations, including limited field of view, variable acoustic windows, difficulty penetrating bone and air, challenges in visualizing extracardiac structures (20), and dependence on operator skill (11). Furthermore, as Apostolopoulou et al. (24) and Haramati et al. (20) pointed out, echocardiographic image quality tends to decline with patients’ age, especially in those who have undergone prior cardiac surgery.
Cardiac catheterization is invasive and carries risks such as hematoma, vascular injury, renal impairment, contrast reactions, radiation exposure, and a very low but notable mortality risk. For these reasons, there has been growing interest in non-invasive methods such as echocardiography and cardiac magnetic resonance (CMR) in recent years (24). However, cardiac catheterization remains a key diagnostic tool in the management of congenital heart disease, as it provides essential hemodynamic data and allows for a more detailed assessment of vascular anatomy (20, 24).
We only compared measurements of pulmonary artery branches and the aortic diameter in echocardiography and cardiac catheterization to determine McGoon and Nakata indices. While our study’s results were consistent with some previous studies, there were notable differences, particularly in not supporting some studies’ recommendation to rely solely on echocardiography for surgical decision-making in these patients. Due to echocardiography’s lower complication rate and its modest correlation with cardiac catheterization for certain indices, we especially recommend using echocardiography for screening. However, this study’s findings suggest that echocardiography alone may not accurately determine the size of the pulmonary branches, and consequently, the Nakata and McGoon indices.
5.1. Conclusions
In patients with ToF, echocardiography alone may not be sufficient for definitive surgical decision-making. While echocardiography remains a safe, accessible, and cost-effective modality, its limitations necessitate the use of cardiac catheterization when precise anatomical measurements are required. However, in cases where echocardiographic images are unclear or equivocal, other methods such as CT angiography should be considered.
5.2. Limitations
Our study had limitations. First, the use of a non-random, purposive sampling method and the lack of blinding of the researchers who performed echocardiography and measured the parameters may limit the generalizability of the findings. Although the calculated minimum sample size was initially set at 65, a total of 120 participants were enrolled to enhance the study’s statistical power. However, in some sections, data were incomplete for a few participants due to various reasons such as lack of cooperation, particularly among younger children, which led to a reduced sample size for certain measurements. Additionally, the wide age and weight range of participants may have affected imaging quality, particularly in older and heavier patients, who often presented with suboptimal acoustic windows in this study. Finally, the study did not include advanced imaging modalities such as CMR, which may provide more detailed and reproducible assessments of pulmonary artery anatomy. Future research should address these limitations by adopting randomized sampling strategies, incorporating blinding protocols, and including larger, stratified patient populations. Comparative studies that integrate CMR or CT angiography may also improve diagnostic accuracy and validate echocardiographic findings more robustly.