1. Background
Atherosclerosis is a chronic inflammatory disease characterized by the development of plaques in the arteries, leading to a decrease in their diameter and ultimately causing blockages in blood flow. These changes progress insidiously and typically manifest with age (above 50 years) as cardiovascular and cerebrovascular events (1). An estimated 28% (approximately 1,067 million) of the global population is affected by carotid atherosclerosis, which is considered the primary cause of mortality due to cardiovascular events (2). In Pakistan, the mortality rate due to these events is estimated to be 20% (around 6.5 million) (3). These figures are alarmingly high and warrant the attention of concerned authorities to take appropriate measures.
Atherosclerosis is a heterogeneous disorder with an unknown root cause (4). Osteopontin (OPN) is an inflammatory cytokine known to play a crucial role in the development of this disease. It is an acidic glycoprotein with a size ranging from 41 to 75 kDa, depending on its various isoforms (5). Alternative splicing of OPN produces multiple isoforms in humans and mice (6). Several studies have supported the linkage between OPN and atherosclerosis. Higher expression of OPN has been reported in cardiovascular issues such as angina pectoris and associated mortality (7). In hypertensive patients with atheroma plaque, significant co-localization of macrophages with OPN was observed (8). Osteopontin also increases the production of reactive oxygen species (ROS) in vascular cells, thus promoting atherogenesis (9). Furthermore, adventitial fibroblasts demonstrate the expression and production of OPN in response to growth factors such as angiotensin II and aldosterone (10).
Statins, including atorvastatin, fluvastatin, pravastatin, rosuvastatin, and simvastatin, are known to reduce plasma cholesterol levels and are considered effective in the management of atherosclerosis (11). Literature has revealed that simvastatin decreases plasma OPN levels (12), affects atheroma plaque morphology (13), and produces pleiotropic effects (14). However, simvastatin faces potential pharmacokinetic challenges such as extensive first-pass effect (15), low aqueous solubility (16), and poor distribution to vascular cells (17). The solution to many of these issues lies in the field of nanomedicine and drug delivery systems. Through its application, the bioavailability of simvastatin in hepatocytes and penetration into vascular cells has been reported to be enhanced, leading to increased efficacy (18) and reduced toxicity (19). Nanoparticles with antioxidant properties may improve vascular dysfunction associated with atherosclerosis (20). Metallic nanoparticles, especially silver (AgNPs), are known for their cardioprotective effects. They decrease ROS production and may reduce ROS-induced NF-κB expression, indicating their protective effect in the cardiovascular system (21). An in vivo study using the ApoE-/- mouse model of atherosclerosis found that among three types of simvastatin nano-formulations (high-density lipoprotein, polymeric micelles, and liposomes), micelles were most effective in reducing macrophage burden in advanced atheroma (22).
Folic acid (FA), also known as vitamin B9, conjugation is considered useful for anti-inflammatory drugs because its receptors are highly expressed in activated macrophages, the crucial immune cells involved in the pathogenesis of inflammatory disorders (23). It is noted that fibroblast cells cultured from humans have folic acid (FRα) receptors in their cell membranes, and enhanced expression is observed on fibroblasts derived from diseased models (23). The lower immunogenicity and cost, along with higher specificity for disease, make FA a favorable molecule for drug conjugation (24). Considering the above, we hypothesize that nano-formulated simvastatin will reduce OPN expression more effectively than conventional simvastatin.
2. Objectives
The NCD report, "Advancing the Global Agenda on Prevention and Control of Non-Communicable Diseases 2000 to 2020: Looking Forwards to 2030", emphasizes the need to identify strategies for achieving targets related to the control of risk factors, diagnosis, and management of cardiovascular diseases to reduce mortality and morbidity. In this context, our research provides a novel approach to drug formulation for the prevention of atherosclerosis. The present study aimed to assess the effectiveness of a folate-conjugated simvastatin silver nano-formulation against a cellular model of atherosclerosis.
3. Methods
3.1. Animals
Male Wistar rats (6 - 8 weeks) were obtained from the animal resource facility of Dow University of Health Sciences. They were housed under standard conditions, including a 12-hour dark/light cycle, a temperature of 25 ± 1°C, and ad libitum access to food and water. The experiment was performed in accordance with the university’s ethical guidelines, which comply with the criteria outlined in the "Guide for the Care and Use of Laboratory Animals" prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23, revised 1985). The study approval number granted by the ethical review board for animal research and ethics is IRB-28/DUHS/Approval/2023/53.
3.2. Chemicals
Phosphate-buffered saline, simvastatin, aldosterone, MTT (3-[4,5-dimethylthiazole-2-yl]2,5-diphenyltetrazolium bromide), dihydroethidine (DHE), trypsin, and DMSO were obtained from Sigma Aldrich (USA). DMEM media, fetal bovine serum, and antibiotics were sourced from Thermo Fisher Scientific (USA). The simvastatin-loaded lecithin nanoparticle (L-SMVNPs) surface coated with folic acid silver nanoparticles (FA-AgNPs) was provided by our collaborator, Professor Dr. Raza Shah, from the Center for Bioequivalence Studies and Clinical Research (C.B.S.C.R), International Center for Chemical and Biological Science (ICCBS), University of Karachi, Karachi, Pakistan.
3.3. Synthesis of Folate-Conjugated Simvastatin Silver Nano-formulation
The AgNPs were synthesized via a chemical reduction method in which FA was used as a reducing and capping agent. Silver nitrate solution was added to an ice-cold FA solution. The solution mixture was stirred on a magnetic stirrer to obtain FA-AgNPs. To remove excess silver ions and free FA molecules, the colloidal AgNPs were centrifuged. Simvastatin was then added to the AgNPs colloidal solution and stirred on a magnetic stirrer at room temperature. The solution was centrifuged to collect the FA-SMV-AgNPs conjugate (25).
3.4. Characterization
The characterization of AgNPs and FA was carried out using a UV-spectrophotometer. The mean diameter and zeta potential of AgNPs and FA-SMV-AgNPs were measured by a DLS analyzer. Microscopic analysis was performed to identify the morphology and particle size of AgNPs and FA-SMV-AgNPs. The characterization and stability work is not yet published.
3.5. Cytotoxicity Assay
Adventitial fibroblast subpopulations obtained from the thoracic aorta of male Wistar rats (6 - 8 weeks) were used for this assay (26). After euthanasia, the thoracic aorta was dissected, cleaned, and the adventitia was obtained by scraping. This isolated adventitia was subsequently washed with PBS (3x), cut into 1 - 2 mm flat segments, and plated on dishes. The medium (DMEM plus 10% FBS and antibiotics) was added, followed by incubation for 3 days. After the addition of fresh medium, the cells were incubated for another 3 days, and the morphological characteristics of the fibroblast cells were observed under a microscope. Upon confirmation, the confluent cells were harvested using 0.25% trypsin and grown in 96-well plates. The treatments were as follows:
- Fibroblast cells alone
- Fibroblasts with aldosterone (10 μM)
- Fibroblasts treated with spironolactone (different doses: 1 - 10 μM) and aldosterone (10 μM)
- Fibroblasts treated with simvastatin (different doses: 1 - 10 μM) and aldosterone (10 μM)
- Fibroblasts treated with folate-conjugated Ag nano-formulation of simvastatin (different doses: 1 - 10 μM) and aldosterone (10 μM)
After these treatments, the fibroblasts were incubated for 24 hours, followed by assessment of cellular viability using the MTT assay. Briefly, MTT was added (20 µL, 5 mg/mL) and incubated for 4 hours, followed by the addition of DMSO (100 µL) in each well. Shaking was performed to dissolve the formazan crystals, and OD (570 nm) was measured using a spectrophotometer.
3.6. Microscopy
After various treatments, the fibroblasts were observed for morphological changes using a microscope (BS-2093A Inverted Microscope, China).
3.7. Osteopontin Expression
The expression of OPN in the fibroblasts following the aforementioned treatments was estimated using qPCR. RNA isolation was performed using TRIzol reagent, followed by cDNA synthesis. Further quantification was done by real-time PCR. A 25 µL reaction mixture was prepared, consisting of cDNA, SYBR Green master mix, and primers (sequences provided in Table 1). The PCR cycle initially involved denaturation for 15 minutes at 95°C. Product amplification was performed at 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds for a total of 30 - 44 cycles. The final extension was done at 72°C for 5 minutes. GAPDH was used as the housekeeping gene. The primer sequences are tabulated in Table 1.
| Genes | Primer Sequences |
|---|---|
| OPN | F - AGGAAGCCAGCCAAGGTAAG |
| R - GGTCCCTCAGAATTCAGCCAG | |
| GAPDH | F - GGAAAGCTGTGGCGTGATGG |
| R - GTAGGCCATGAGGTCCACCA |
3.8. Osteopontin Quantification
The OPN quantification was estimated in fibroblast lysates using an ELISA kit (Invitrogen rat OPN ELISA kit, ERA46RB). Briefly, the treated cells were lysed, and debris was removed through centrifugation. The cell lysate was added to pre-coated plates (biotinylated anti-OPN antibody) as described in the manufacturer’s protocol. After washing away unbound antibody, horseradish peroxidase (HRP)-conjugated streptavidin and tetramethylbenzidine (TMB, chromogenic substrate) were added, followed by measurement of optical density (OD) at 450 nm.
3.9. Reactive Oxygen Species Quantification
To estimate ROS in the treated fibroblasts, DHE was used as a fluorescent probe. After treatment, the cells were incubated with the dye (5 μM) in the dark, followed by detection of fluorescence at an excitation wavelength of 520 nm and an emission wavelength of 605 nm.
3.10. Statistical Analysis
Data are presented as mean ± SEM (n = 3 per group). Statistical differences among various means were measured using one-way ANOVA followed by post hoc analysis (Bonferroni post hoc test) using SPSS software (version 19.0, SPSS Inc., Chicago, IL). The Shapiro-Wilk test was used to assess the normality of data distribution, and Levene’s test was applied to evaluate the homogeneity of variances across groups. All data met the required assumptions, thereby justifying the use of ANOVA.
4. Results
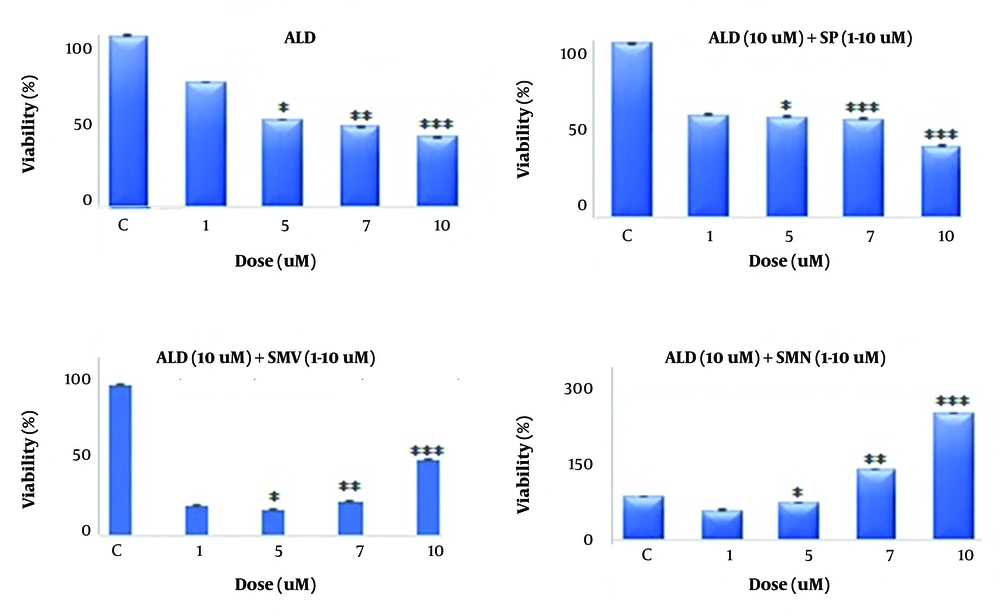
4.1. Cytotoxicity Assay
Aldosterone treatment caused a significant dose-dependent decrease in the viability of fibroblasts compared to the control (Figure 1). At the highest tested dose of 10 μM, it caused a 50% reduction in viability (P < 0.001). Spironolactone treatment provided mild protection against aldosterone-induced toxicity, while simvastatin and its nano-formulation exhibited a dose-dependent increase in cell viability, with the latter being superior (300% at the highest tested dose of 10 μM; P < 0.001) in exerting this beneficial effect.
The figures depict the viability (percent mean ± SEM) of fibroblast cells treated with aldosterone, spironolactone, simvastatin, and its nano-formulation. A dose-dependent decline in cell viability was observed after treatment (1 - 10 μM) with aldosterone compared to the control (fibroblast alone). In the presence of aldosterone (10 μM), co-administration (1 - 10 μM) of spironolactone demonstrated mild protection, while simvastatin and its nano-formulation demonstrated a remarkable protective effect. The percent viability in the nano-formulation group was higher than simvastatin alone. Asterisks indicate statistical differences (* P < 0.05, ** P < 0.01, and *** P < 0.001) compared to the control group.
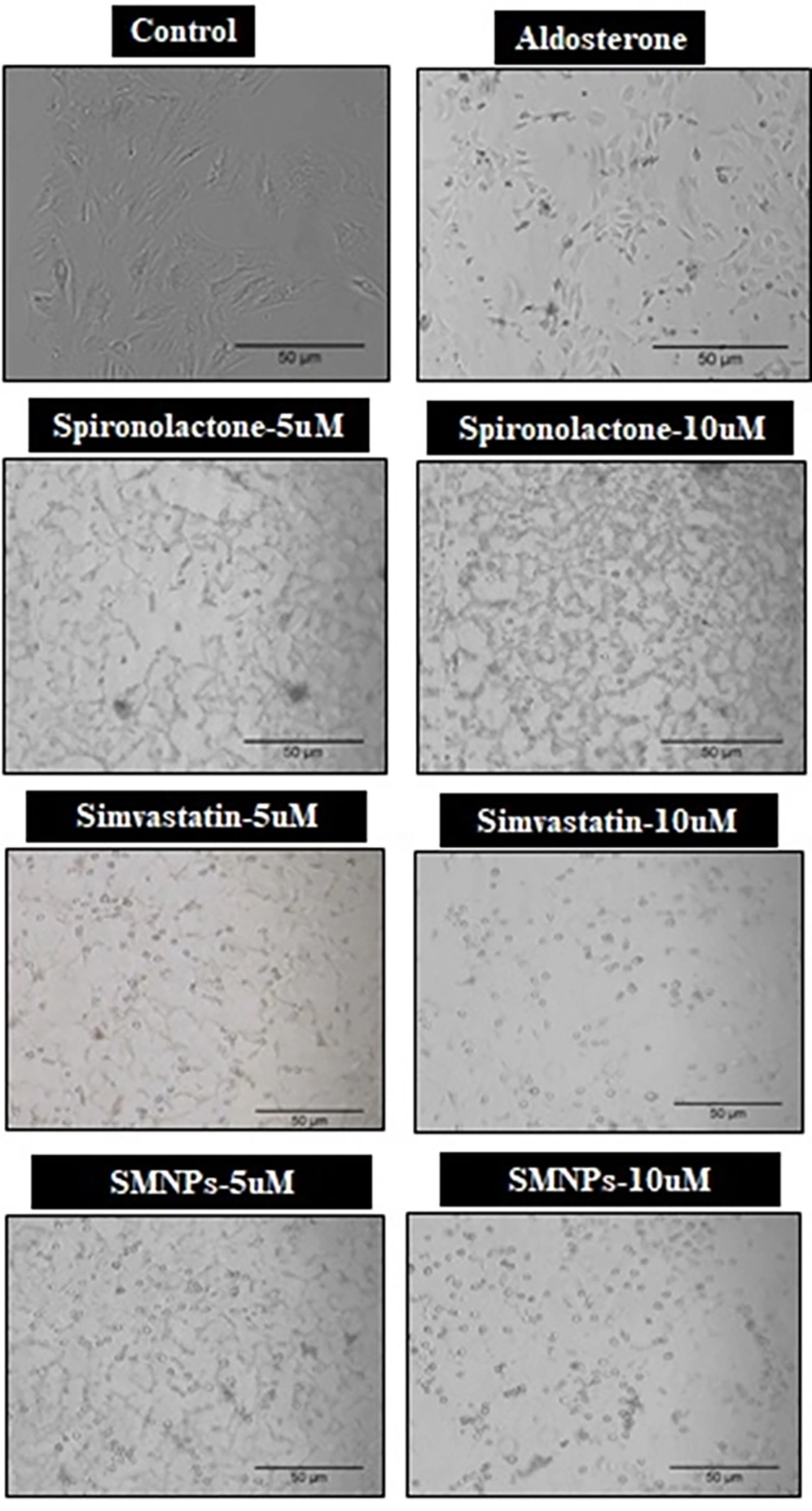
4.2. Microscopy
Microscopic observation exhibited a remarkable reduction in cell viability along with altered cellular architecture following treatments with aldosterone and spironolactone (Figure 2). However, the cells were preserved in the case of simvastatin and its nano-formulation.
The image depicts the decline in cell viability and shape, along with the presence of debris in aldosterone and spironolactone-treated cells. However, the cells remained intact in the simvastatin and its nano-formulation (SMNP) treated groups.
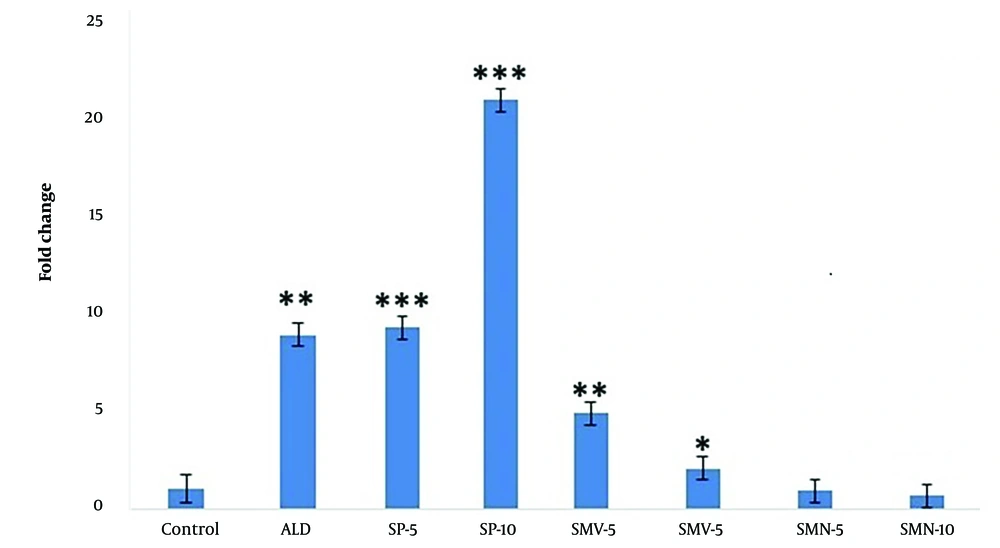
4.3. Osteopontin Expression
Aldosterone treatment caused a significant increase (P < 0.01, 9x) in OPN expression compared to control cells (Figure 3). The levels remained significantly high (P < 0.001) upon co-administration of spironolactone. Simvastatin treatment caused a dose-dependent decrease in OPN expression [5x (P < 0.01) and 2x (P < 0.05) at doses of 5 and 10 μM, respectively], while in the case of its nano-formulation, the levels were similar to those of control cells.
The bar graph exhibits a significant increase in OPN expression (fold change mean ± SEM) in fibroblasts following treatments with aldosterone (ALD 10 μM) compared to control cells. In the presence of ALD, spironolactone (SP 5 and 10 μM) treated cells enhanced OPN expression. However, simvastatin (SMV 5 and 10 μM) and its nano-formulation (SMN 5 and 10 μM) significantly blocked the aldosterone-induced rise in OPN levels. Asterisks indicate statistical differences (* P < 0.05, ** P < 0.01, and *** P < 0.001) compared to control.
4.4. Osteopontin Quantification
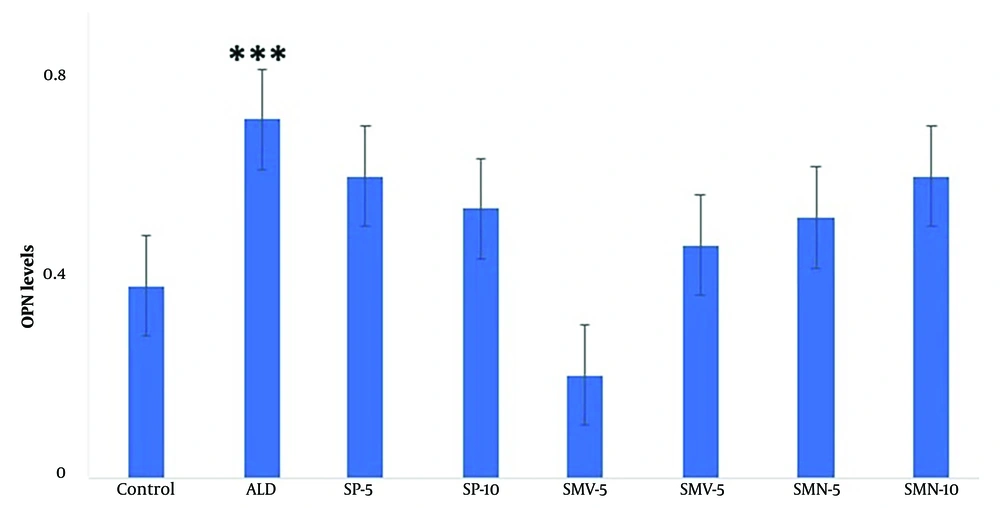
Aldosterone treatment significantly (P < 0.001) raised OPN levels in fibroblasts compared to the control (Figure 4). However, no significant alteration was observed in the rest of the treatment groups.
The graph depicts OPN levels (mean ± SEM) in fibroblasts. Among various treatment groups, OPN levels were significantly elevated in the aldosterone treatment group alone compared to control. The rest of the treatments, i.e., SP (5 and 10 μM), SMV (5 and 10 μM), and its nano-formulation (SMN 5 and 10 μM), significantly blocked the aldosterone-induced increase in OPN levels. Asterisks indicate statistical differences (*** P < 0.001) compared to control.
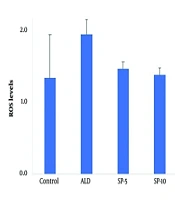
4.5. Reactive Oxygen Species Quantification
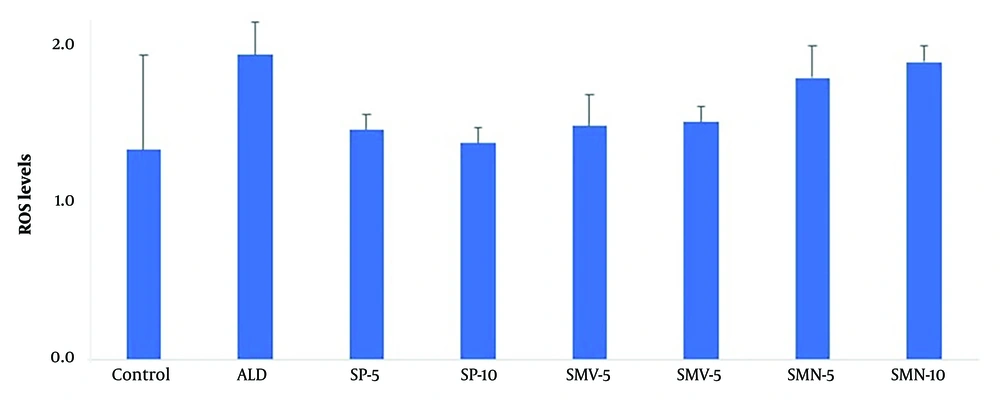
The ROS levels remained normal in all treatment groups compared to control (Figure 5).
The graph exhibits ROS levels (mean ± SEM) in fibroblasts following various treatments. None of the treatment groups, i.e., aldosterone (ALD) alone or in combination with spironolactone, i.e., spironolactone (SP 5 and 10 μM), simvastatin (SMV 5 and 10 μM), and its nano-formulation (SMN 5 and 10 μM), significantly altered ROS levels in fibroblasts.
5. Discussion
Atherosclerosis is considered the leading cause of mortality worldwide. Osteopontin is a cytokine reported to play a pivotal role in the development of atherosclerosis. Simvastatin is a lipid-lowering drug with a reported ability to decrease OPN levels but possesses unfavorable pharmacokinetic characteristics. In view of this, the study aimed to assess the effectiveness of a nano-formulation of simvastatin in a cellular model of atherosclerosis, specifically aldosterone-stimulated adventitial fibroblast cells.
Our data showed that aldosterone, a steroidal hormone of the adrenal gland, significantly reduced the viability of fibroblasts compared to the control (Figure 1). Microscopic observation also revealed a similar outcome (Figure 2). Previous work has shown that aldosterone increases OPN expression in these fibroblasts (10). Similarly, our data revealed increased transcription (Figure 3) and translation (Figure 4) of OPN following aldosterone treatment. Furthermore, OPN has been reported to induce inflammation in the vasculature by increasing ROS (9). Contrary to this, our data did not reveal any sign of oxidative stress compared to the control (Figure 5). Previous studies suggest that OPN-induced ROS generation is reversible in acute scenarios (7). It is noteworthy that we measured ROS 24 hours after aldosterone exposure. Therefore, the resolution of acute changes over time may underlie our results. This is supported by the entire data set, where none of the treatment groups altered ROS levels. However, further work is required to delineate this outcome.
Literature reveals that aldosterone-induced OPN expression is mediated via mineralocorticoid receptors (27). Hence, spironolactone, a mineralocorticoid receptor antagonist, was used to confirm this notion. Notably, spironolactone did not offer much protection to fibroblasts in the presence of aldosterone, as exhibited by viability and microscopic data sets. Therefore, it can be deduced that aldosterone-induced OPN expression noted in the present study was independent of mineralocorticoid receptor involvement. This notion is further supported by increased transcription while unaltered OPN protein levels in the spironolactone-treated group.
Our viability and microscopic data regarding simvastatin and its nano-formulation revealed a dose-dependent increase in fibroblast viability. In the case of the nano-formulation, an extremely remarkable increase in the cell population, even beyond the control group, was noted. This suggests enhanced distribution of simvastatin in its nano-formulation form compared to its conventional form. It appears that nano-engineering and folate conjugation have likely enhanced the efficacy of simvastatin. Earlier reports revealed that simvastatin has the ability to proliferate mesenchymal stem cells by upregulating cell cycle regulators, proliferation markers, and anti-apoptotic gene expression (28). Since, in our study, fibroblasts were obtained from adventitia, there is a possibility that our cell sample may also contain stem cells (29). Hence, the widespread cellular proliferation observed could likely be due to the presence of stem cells along with fibroblasts, which extensively divide in the presence of simvastatin.
Furthermore, simvastatin and its nano-formulation significantly reduced OPN gene expression. Notably, the nano-formulation was found to be superior to the conventional molecule in downregulating this gene, which again supports the beneficial impact of nano-engineering on the pharmacokinetic profile of simvastatin, especially cell penetration (30). However, this downregulation observed at the transcription level was not obvious in the case of OPN translation. Although a decrease was noted in its levels, it was statistically non-significant compared to the control. Literature exhibits that mRNA levels do not correlate with the levels of numerous protein isoforms, whose abundance is primarily regulated by post-translational phenomena (31). Osteopontin has been reported to possess various isoforms (6). Moreover, there are multiple levels of regulation between transcription and translation, which work independently and may affect the relative quantities of mRNA and protein to varying degrees. Additionally, the rate of protein degradation can also influence the mRNA and protein association (32). However, further work is required to delineate this unusual outcome regarding OPN levels.
Although the present study provides initial proof-of-concept (in vitro), the primary limitation is the lack of in vivo data assessing the efficacy of the nano-formulation in an animal model of atherosclerosis. Moreover, clinical studies are needed to establish their use in humans. Furthermore, particle size reduction affects the physicochemical characteristics of chemicals, which may significantly impact the safety of the medicine. Hence, the safety of this new formulation needs to be established. Cost-effectiveness is yet another issue warranting the attention of developers. Although the production cost of the nano-formulation is higher, its lower side effects and better efficacy lead to lower treatment costs compared to the conventional molecule. Future studies will aim to address these limitations to translate the findings to bedside applications.
5.1. Conclusions
It can be deduced that simvastatin, especially its nano-formulation, demonstrated a protective effect in the cellular model of atherosclerosis, which can be attributed to decreased OPN expression and better cellular distribution. Hence, it presents itself as a favorable candidate for an anti-atherosclerosis drug discovery program.