1. Background
Coronavirus infection, caused by SARS-CoV-2, developed in December 2019, rapidly reached pandemic proportions and led to significant changes in clinical practice and scientific research (1-6). There was an initial assumption that children are less susceptible to COVID-19. Nevertheless, a relevant literature review confirms not only respiratory but also extrapulmonary symptoms in children, including cardiovascular manifestations (5-11). The mechanism of virus penetration is facilitated by angiotensin-converting enzyme 2 (ACE2), which acts as the functional receptor for the virus. The ACE2 is widely present in various organs, including the heart and vascular endothelium (12-15). The discovery of SARS-CoV-2 entry made it possible to explain the multiorgan targets, since the ACE2 protein is expressed in the respiratory tract, heart and vascular endothelium, intestinal epithelium, and kidneys (12-14, 16). Studies show that children aged over 10 years, especially those with concomitant pathologies, are more likely to develop cardiac damage while having COVID-19. However, its signs and symptoms are mostly mild and nonspecific, which complicates the diagnosis (8-11, 16). Of particular importance are complications such as myocarditis, heart failure, arrhythmia, and thromboembolic events observed in adults, but these have not been sufficiently studied in childhood (11, 16-18),(19-22).
According to theoretical reviews and clinical experience, children of any age are vulnerable to virus penetration. There is a group of patients (7.3 - 13.6%) who exhibit a wide range of clinical manifestations, from asymptomatic variants to multiple organ failure, culminating in a number of cases with a fatal outcome (2, 20, 21, 23, 24). In pediatric practice, the systematic study of clinical and functional characteristics of cardiovascular complications remains unresolved, especially in the Republic of Kazakhstan, where relevant studies are sporadic. The lack of data on children’s post-COVID-19 rehabilitation creates significant gaps in understanding effective methods of health maintenance (4, 5, 20, 23-26). This research aims to examine the characteristics of cardiovascular damage in children diagnosed with COVID-19. The focus is on finding solutions for comprehensive rehabilitation, emphasizing its scientific novelty and practical significance. The results can form the basis for new approaches to diagnostics, treatment, and recovery of post-COVID-19 children with cardiovascular complications. This is especially relevant given the ongoing impact of the pandemic (4, 5, 15, 25-27).
2. Objectives
The aim of the research is to study the clinical and functional state of the cardiovascular system in children with a history of COVID-19.
3. Methods
The study was conducted at pediatric healthcare facilities in Aktobe, Kazakhstan, from 2023 to 2024.
3.1. Study Design
The one-time cross-sectional study was divided into three stages.
The first stage involved a retrospective study of 400 pediatric medical records with a confirmed diagnosis of COVID-19 from October to December 2020 in the Regional Infectious Diseases Hospital. Inclusion criteria: Children from One to 18 years old with a history of PCR-confirmed COVID-19; exclusion criteria: Children without laboratory confirmation of COVID-19.
At the second stage, children were examined in the long-term follow-up. The total sample comprised 400 children with PCR-confirmed COVID-19, enabling broad age representation and providing sufficient statistical power to identify general patterns and assess the frequency of clinical-functional alterations. Children with congenital malformations, genetic syndromes, or chronic somatic pathology that may affect the cardiovascular system were excluded. Finally, 228 children made up the main group. The control group included children with respiratory symptoms during the pandemic, but COVID-19 was not laboratory confirmed (n = 172). The compared groups were equivalent by gender and age. The follow-up examination was conducted after 24 - 36 months in 2023 - 2024. All the examined children were divided into age groups (Table 1).
| Age Groups (y) | Main Group | Control Group | ||||
|---|---|---|---|---|---|---|
| N | Girls | Boys | N | Girls | Boys | |
| 4 - 7 | 53 | 25 | 28 | 34 | 15 | 19 |
| 8 - 11 | 25 | 8 | 17 | 29 | 7 | 22 |
| 12 - 15 | 63 | 27 | 36 | 33 | 13 | 20 |
| 16 - 18 | 87 | 39 | 48 | 76 | 39 | 37 |
| Total | 228 | 99 | 129 | 172 | 74 | 98 |
Clinical examinations in the second stage included: Past medical history, assessing complaints such as cardiac pain, shortness of breath, dizziness, fainting, arrhythmia, etc., and results of physical examination methods. Simultaneously, all children underwent blood pressure (BP) measurement, complete blood count (CBC), urinalysis, and electrocardiogram (ECG). The third stage involved a more in-depth clinical assessment. The 40 children included in stage 3 were purposefully selected from the main group based on objective cardiovascular abnormalities identified during the stage 2 follow-up: Persistent cardiovascular symptoms, ECG abnormalities (e.g., PQ interval shortening ≥ 9.3%, QT prolongation, ventricular extrasystoles), and BP irregularities. The selection was clinically justified and aimed at a more detailed study of possible cardiac disorders in the most vulnerable subjects. These children underwent immunological and biochemical examinations for lactate dehydrogenase (LDH), ferritin, pro-BNP, and C-reactive protein (CRP). A coagulogram was conducted to examine activated partial thromboplastin time (APTT). The immunological examination identified immunoglobulin G (IgG) antibodies to SARS-CoV-2 as follows:
1. "Less than 0.9 PI": Not detected.
2. "0.9 - 1.0 PI": A questionable result, the study should be repeated after 10 days.
3. "Greater than 1.1 PI": Positive result.
Instrumental methods included cardiac ultrasound, 12-lead Holter monitoring, and 24-hour BP monitoring. The standard BP parameters studied were average values (systolic, diastolic, mean BP, and pulse rate) per 24 hours, during the day (wake period), and at night (sleep period); pressure load values (Time Index, Measurement Index) for systolic blood pressure (SBP) and diastolic blood pressure (DBP). The BP variability was assessed by the standard deviation from the mean BP per day during the day and night periods. The daily rhythm of BP (Daily Index), the degree of night-time BP decrease (DNBP), morning blood pressure (MBR) increase, and the rate of morning rise (RMR) were assessed.
To increase the reliability of the results and eliminate potential bias, the following measures were taken: Inclusion and exclusion criteria were strictly observed, ensuring homogeneity of the sample in terms of clinical and demographic characteristics. A retrospective analysis of medical records was performed using a standardized data collection form, minimizing the influence of subjective factors. All clinical, laboratory, and instrumental examinations were performed according to unified standards and on certified equipment. The interpretation of the results (in particular, ECG) was performed by specialists unaware of the patients' clinical history, which excluded observational bias. The sample in the third stage was formed exclusively based on objective medical indications, omitting socioeconomic factors.
3.2. Statistics
The data were consolidated into a single dataset in Microsoft Excel 2016, which included information from the individual registration forms and laboratory test results. Statistical analyses were performed using IBM SPSS Statistics, version 25. Descriptive statistics, including medians, interquartile ranges (IQR), frequencies, and percentages, were used to characterize clinical variables. The normality of the distribution of quantitative variables was tested using the Shapiro-Wilk test. As most variables deviated from a normal distribution (P < 0.05), nonparametric methods were applied for further analyses. The Mann-Whitney U test was used to evaluate group differences. Tests were two-tailed, and results were considered statistically significant at P < 0.05 and P < 0.001.
The study was approved by the Local Ethics Committee of West Kazakhstan Marat Ospanov Medical University, Aktobe (protocol #8, dated October 20, 2022).
4. Results
The analysis of medical records of the study group (n = 228) demonstrated an obvious predominance of boys, with 147 (64.75%), while the number of girls was 81 (35.25%). There was a prevalence of mild (50.5%) and moderate (43%) courses of coronavirus infection, while severe cases were documented in 6.5% of instances. The relationship between age and the course of COVID-19 revealed a clear dominance of adolescent children in all clinical variants of the disease.
The polymorphism and non-specificity of clinical manifestations in children were revealed. In clinical syndromology, cough (75.5%), sneezing and rhinorrhea (35.5%), and dyspnea (10%) were noted with the highest frequency in the respiratory system. In the gastrointestinal tract, appetite disorders (30%), diarrhea (29.75%), and vomiting and nausea (21.75%) were noted. There were symptoms of intoxication: Weakness (57.75%), headache (35%), anxiety, and sleep disorders (33.75%) together with fever. In fewer clinical observations, symptoms such as dizziness (7.25%), enanthema (5.75%), and convulsions (3.75%) were described. Olfactory disorders occurred in children only in 2.5% of cases.
Regarding the cardiovascular system, tachycardia was observed in 74%, cardiac pain in 9.25%, and increased BP in 7.25% of cases. Cardiovascular changes were observed in the acute period of the disease, occurring at the height of intoxication with subsequent rapid relief. The connection of cardiologic symptoms with the severity of the disease was not revealed.
The results of laboratory indicators indicate no specific hematologic modifications. In some cases, there was an increase in white blood cells (WBCs) and lymphocytes, along with an acceleration of erythrocyte sedimentation rate (ESR). However, average hemogram indicators in all age groups were within reference values and corresponded to the hematologic reaction to a viral infection. At the second stage, 228 children with a history of COVID-19 were examined. The control group consisted of 172 children with epidemiologically confirmed exposure and respiratory symptoms, but without laboratory confirmation of COVID-19. All children underwent clinical and laboratory examinations, including the collection of complaints, anamnestic data, objective examination, BP measurement, CBC, urinalysis, and ECG (Table 2).
| Symptoms | Main Group (N = 228) | Control Group (N = 172) | ||||||
|---|---|---|---|---|---|---|---|---|
| 4 - 7 (N = 53) | 8 - 11 (N = 25) | 12 - 15 (N = 63) | 16 - 18 (N = 87) | 4 - 7 (N = 34) | 8 - 11 (N = 29) | 12 - 15 (N = 33) | 16 - 18 (N = 76) | |
| Cardiac pain | - | 2 (8) | 4 (6.3) | 6 (6.8) | - | - | 1 (3) | 1 (1.3) |
| Dysrhythmia | - | 3 (12) | 5 (7.9) | 7 (8) | - | 1 (3.4) | 1 (3) | 1 (1.3) |
| Increased fatigue | - | 2 (8) | 4 (6.3) | 4 (4.5) | - | - | 1 (3) | 2 (2.6) |
| Sternal discomfort | - | 1 (4) | 2 (3.2) | 2 (2.3) | - | - | - | 1 (1.3) |
| Incomplete inhalation feeling | - | 1 (4) | 3 (4.7) | 5 (5.7) | - | - | - | 2 (2.6) |
| Reccurent abdominal pain | - | 2 (8) | 3 (4.7) | 5 (5.7) | - | 1 (3.4) | 1 (3) | - |
| Pulsing headache with nausea | - | - | 2 (3.2) | 6 (6.8) | - | - | 1 (3) | 2 (2.6) |
| Poor tolerance to physical activity | - | 3 (12) | 4 (6.3) | 7 (8) | - | - | - | 2 (2.6) |
| Cold hands | - | 4 (16) | 3 (4.7) | 5 (5.7) | - | 1 (3.4) | 1 (3) | 1 (1.3) |
| Allergicreactions (angioedema, urticaria) | 5 (9.4) | 2 (8) | 1 (1.5) | 5 (5.7) | - | - | 1 (3) | 2 (2.6) |
| Palm hyperhidrosis | - | 1 (4) | 3 (4.7) | 4 (4.5) | - | - | 1 (3) | 1 (1.3) |
| Tendencytoskinredness | - | 3 (12) | 5 (7.9) | 7 (8) | - | - | 2 (6) | 2 (2.6) |
a Values are expressed as No. (%).
As can be seen from Table 2, adolescents in the main group more often had complaints of cardiac pain, arrhythmia, increased fatigue, and poor tolerance to physical activity. Hematologic parameters of both groups corresponded to reference values, and there was no significant difference in mean values between the groups. Analysis of the average heart rate in the main and control groups showed compliance with reference values. Comparative data of ECG parameters are presented in Table 3.
| Age Groups (y) | Heart Rate (Main Group) | Heart Rate (Control Group) | Р-Value | Р Wave (Main Group) | Р Wave (Control Group) | Р-Value | PQ (Main Group) | PQ (Control Group) | Р-Value | Qrs (Main Group) | Qrs (Control Group) | Р-Value | QT (Main Group) | QT (Control Group) | Р-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 - 7 | 103 (100 - 117.5) | 100 (96 - 120) | 0.2 | 0.07 (0.06 - 0.08) | 0.08 (0.06 - 0.08) | 0.59 | 0.12 (0.12 - 0.14) | 0.12 (0.12 - 0.14) | 0.66 | 0.06 (0.06 - 0.075) | 0.06 (0.06 - 0.08) | 0.48 | 0.36 (0.36 - 0.375) | 0.36 (0.36 - 0.36) | 0.63 |
| 8 - 11 | 91.5 (84.5 - 100.75) | 90 (88 - 111) | 0.27 | 0.08 (0.06 - 0.08) | 0.08 (0.06 - 0.08) | 0.57 | 0.13 (0.12 - 0.145) | 0.135 (0.12 - 0.14) | 0.85 | 0.06 (0.06 - 0.07) | 0.06 (0.06 - 0.06) | 0.3 | 0.36 (0.36 - 0.36) | 0.36 (0.36 - 0.36) | 0.75 |
| 12 - 15 | 90 (78 - 100) | 86 (79 - 96) | 0.56 | 0.08 (0.06 - 0.08) | 0.08 (0.06 - 0.08) | 0.36 | 0.13 (0.10 - 0.16) | 0.14 (0.12 - 0.16) | 0.83 | 0.06 (0.06 - 0.08) | 0.06 (0.06 - 0.065) | 0.33 | 0.36 (0.36 - 0.38) | 0.36 (0.36 - 0.375) | 0.74 |
| 16 - 18 | 78 (70 - 88) | 76 (70 - 86) | 0.67 | 0.08 (0.06 - 0.08) | 0.08 (0.06 - 0.08) | 0.38 | 0.13 (0.11 - 0.16) | 0.14 (0.12 - 0.16) | < 0.05 | 0.08 (0.06 - 0.08) | 0.06 (0.06 - 0.08) | 0.18 | 0.36 (0.36 - 0.36) | 0.36 (0.36 - 0.38) | 0.88 |
As can be seen from Table 3, the main parameters of waves and intervals were within reference values. However, 14 children (9.3%) aged 12 - 18 years in the main group exhibited relative shortening of the PQ interval, while in the control group, only 6 (5.5%) children showed this. Comparative data of BP for the main and control groups are presented in Table 4.
| Age Groups (y) | Main Group | Control Group | ||
|---|---|---|---|---|
| SBP | DBP | SBP | DBP | |
| 4 - 7 | 100 (92.5 - 110) | 60 (60 - 70) | 107 (100 - 114) | 60 (60 - 70) |
| 8 - 11 | 113 (106 - 118) | 68 (62 - 72) | 112 (105 - 118) | 67 (61 - 72) |
| 12 - 15 | 118 (110 - 130)* | 71 (70 - 80) | 115 (109 - 120) | 71 (68 - 80) |
| 16 - 18 | 125 (118 - 135)* | 75 (70 - 84) | 120 (110 - 130) | 73 (70 - 84) |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure.
The BP parameters in both groups are within the limits of age norms. However, as can be seen from Table 4, there is a tendency for an increase in the main group.
At the final stage of the study, children who exhibited clinical and instrumental signs of cardiovascular disorders (complaints, ECG changes, and BP abnormalities) were examined. The age composition of the described group included preschool (8/20%), school (9/22.5%), and adolescent (23/57.5%) children. The structure of the adolescent group was represented by children aged 12 - 15 years (17/42.5%) and 16 - 18 years (6/15%). Immunoglobulin M (IgM) was not detected in all age groups, indicating the absence of acute illness. The IgG levels were significantly higher than reference values for healthy children, as the immune response leads to an increase in antibody levels, including IgG.
In the group of children aged 4 - 7 years, the average level of IgG is 222.04 g/L, with a reference range of 4 - 9 g/L. This indicates a pronounced immune response. In the group of 8 - 11 years old, the average level of IgG is 121 g/L (standard is 5 - 14 g/L). Compared to other groups, there is a slight decrease. The lowest individual index (47.26 g/L) also belongs to this group, which may indicate a weaker or variable immune response.
In the 12 - 15 years group, the mean IgG level reaches 356.4 g/L (normal is 14 g/L), and the highest recorded figure (994.4 g/L) is also found here. This may indicate an enhanced immune response in this age range. In the 16 - 18 years group, the IgG level averages 480.45 g/L, which is also much higher than the standard (14 g/L). The IgG levels gradually increase with age, reaching a peak in adolescence (12 - 18 years). This may be due to the peculiarities of the formation and maturity of the immune system in children.
Assessment of blood biochemical analysis showed that CRP in all age groups was within reference values, confirming the absence of acute manifestation. Ferritin levels also did not exceed age normative values in any of the studied groups.
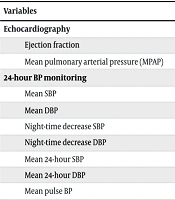
When evaluating APTT, no exceeding of reference values was revealed. To illustrate structural and functional changes in the cardiovascular system, cardiac ultrasound was performed in all children (Table 5). During echocardiography conducted 24 - 36 months after COVID-19, the size of heart cavities, left ventricular ejection fraction, and myocardial thickness in the predominant majority of patients corresponded to the mass-weighted values.
| Variables | 4 - 7 y (N = 8) a | 8 - 11 y (N = 9) a | 12 - 15 y (N = 17) a | 16 - 18 y (N = 6) a |
|---|---|---|---|---|
| Echocardiography | ||||
| Ejection fraction | 67 (65 - 68) | 69 (66.5 - 70.5) | 65 (63.5 - 66.0) | 63.5 (62 - 64.0) |
| Mean pulmonary arterial pressure (MPAP) | 24 (21 - 25) | 23 (22 - 24) | 25 (24 - 27) | 26 (24 - 30) |
| 24-hour BP monitoring | ||||
| Mean SBP | 83 (83 - 85) | 85 (84 - 85.5) | 102 (97.5 - 115) | 103 (92.7 - 110.5) |
| Mean DBP | 60 (50 - 63) | 60 (58 - 61) | 65 (52 - 67) | 60 (57.5 - 66.7) |
| Night-time decrease SBP | 10 (10 - 10) | 10 (6.9 - 11) | 8.2 (6.9 - 9.8) | 5.8 (3.0 - 7.0) |
| Night-time decrease DBP | 10 (10 - 11) | 10 (1.7 - 11) | 12 (10.5 - 12) | 6.9 (2.7 - 10) |
| Mean 24-hour SBP | 84 (82 - 85) | 84 (83 - 84.5) | 99 (95.5 - 105.5) | 101.5 (91 - 119) |
| Mean 24-hour DBP | 59 (49 - 60) | 59 (55 - 60) | 60 (59.5 - 60.5) | 61 (55.2 - 70) |
| Mean pulse BP | 30 (27 - 30) | 29 (28.5 - 30) | 40 (33 - 43.5) | 39.5 (35 - 48) |
| 24-hour ECG monitoring | ||||
| Daytime heart rate | 95 (91 - 111) | 88 (86 - 102) | 93 (84 - 96.5) | 82.5 (78.2 - 91.7) |
| Night time heart rate | 75 (69 - 82) | 65 (62 - 77) | 75 (69.5 - 80) | 56 (53.2 - 68.2) |
| PQ | 0.15 (0.12 - 0.16) | 0.12 (0.12 - 0.15) | 0.14 (0.14 - 0.15) | 0.17 (0.15 - 0.23) |
| QT | 361 (332 - 388) | 391 (371 - 411) | 386 (367 - 418) | 383 (379 - 396) |
| QT corrigated | 438 (420 - 449) | 416 (405 - 428) | 425 (415 - 435) | 413 (393 - 428) |
Abbreviations: BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; ECG, electrocardiogram.
a Q (Q1 - Q3).
When analyzing the echocardiographic examination, the following were detected: An open oval window of 0.2 - 0.3 cm was found in 2 patients (5%) (95% CI: -1.8 to 11.8), and one case (2.5%) with two echo-interruptions of 0.3 and 0.2 cm with discharge from left to right (95% CI: -2.3 to 7.3). In 3 cases (7.5%) (95% CI: -0.7 to 15.7), there was a mitral valve prolapse stage 1. Echocardiography analysis revealed 1 case (2.5%) (95% CI: -2.3 to 7.3) of systolic pulmonary artery pressure (SPAP 32 mmHg) with tricuspid regurgitation of 1.5 mmHg, and 1 child (2.5%) (95% CI: -2.3 to 7.3) had mild left ventricular enlargement. In the last case, there were no clinical manifestations, the ejection fraction was at 71%, and Pro-BNP was within reference values, which was considered a variant of the norm.
Due to BP lability at the previous examination, children underwent 24-hour BP monitoring. It was noted that in the group of children from 4 to 7 years old, the average BP was 85/56 mmHg; in the age group from 8 to 11 years old, it was 86/56 mmHg; and in adolescents, it was 107/64 mmHg (including children from 16 to 18 years old - 100.8/61.8 mmHg). The relative BP decrease in the last group was found due to hypotension of 80/53 mmHg in one child.
Analysis of daily BP dynamics revealed an insufficient night-time decrease in both SBP and DBP in children beginning from school age. At the same time, the percentage of children exhibiting a non-dipper BP profile in the age group from 9 to 11 years was 11.1%, and in adolescents, it was 47.8%, with children up to 15 years at 35.2% and those aged 16 to 18 years at 83.3%. The mean indices of daily ECG monitoring fell within reference values.
5. Discussion
In the study, there was a predominance of boys (64.75%) over girls (35.25%), with a mean age of 9.2 ± 1.16 years. These findings align with the results of a systematic review by Hoste et al., which indicated that COVID-19 cases are more common among boys (16). Gender differences were also found in a study by Du et al., where the majority of patients with COVID-19 were also boys (10). The data emphasize the need to consider gender and age when developing preventive and treatment strategies. Most children had a mild to moderate course of the disease, which coincides with the findings of Patel and the systematic review by Martins et al.: Children, unlike adults, tolerate the infection more easily and show better outcomes (3, 12). A study by Du et al. covering 9,357 students showed a markedly higher rate of asymptomatic infections in children (10).
Involvement of the cardiovascular system in the pathological process is considered an important prognostic factor determining the risk of a complicated course and worse prognosis in manifestations of coronavirus infection in children (28). According to Rodionovskaya et al. and Hoste et al., isolated or combined cardiovascular lesions associated with coronavirus infection are present in only 0.5% of cases, which is due to the peculiarities of the course of the disease in asymptomatic and mild forms (16, 29).
The most common symptoms among children were cough (75.5%), tachycardia (74%), and weakness (57.75%). Dizziness (7.25%) and olfactory disturbance (2.5%) were less common. These findings align with those described by Du et al., which indicated that the predominant symptoms in children were cough and fever (10). The systematic review included ten studies, comprising two case series and eight retrospective medical records reviews, describing a total of 2,914 pediatric patients with COVID-19. Patients tended to have cough (48%), fever (47%), and sore throat/pharyngitis (28.6%) more than upper respiratory tract symptoms/rhinorrhea/sneezing (13.7%), vomiting/nausea (7.8%), and diarrhea (10.1%) (3). The polymorphism of clinical manifestations also confirms the importance of a comprehensive approach to COVID-19 in children. Long-term symptoms such as fatigue and dyspnea persisted in some patients six months after recovery. This is consistent with the description of post-COVID-19 syndrome presented by Mayuga et al. (17).
The majority of patients had no specific changes in laboratory parameters, which coincides with the data of foreign researchers (10, 30). The clinical spectrum of cardiovascular involvement included tachycardia (74%), cardiac pain (9.25%), and elevated BP (7.25%). These alterations predominantly manifested during the acute phase of the illness, occurring at the peak of intoxication with subsequent rapid relief. The connection of cardiologic symptoms with the severity of the disease was not revealed. However, the frequency of cardiovascular manifestations increases substantially in complicated variants of coronavirus infection (severe, extremely severe form of the disease, multisystem inflammatory syndrome) and is registered in up to 79.3% of cases (31).
Adolescents in the main group demonstrated more clinical manifestations, such as cardiac pain, dysrhythmia, increased fatigue, poor tolerance of physical activity, and a tendency toward increased BP, which elevates the risk of cardiovascular complications. The significant differences in the PQ interval observed in the main group of adolescents indicate cardiovascular system involvement in the pathological process, which is an important component of post-COVID syndrome, also known as prolonged COVID-19 (4, 20, 25, 27, 32). This condition can affect one-third of patients with pronounced symptoms of COVID-19. Shortened PQ syndrome is a predictor of paroxysmal tachycardia and other various arrhythmias. The prevalence of shortened PQ is currently 6.9% in the general population. In the study, a relative shortening of the PQ interval was diagnosed in relation to the actual heart rate. The PQ interval shortening associated with COVID-19 requires further study (17).
Elevated IgG levels were observed in all age groups, indicating a persistent immune response. These data are consistent with the findings of Bielecka-Dabrowa et al., who emphasize the importance of the immune response in COVID-19 (22). The association between high antibody titers and persistent symptoms suggests the involvement of an immune mechanism (8). The study of the immune response in children with a history of COVID-19 is an important area of research. The level of IgG antibodies is significantly higher than the reference values for healthy children, indicating the formation of immunity after infection. However, data on the persistence of this immunity are contradictory: Some studies indicate a decrease or disappearance of IgG 3 - 6 months after the disease (33). In this case, the increased values of serum IgG concentration 24 - 36 months after COVID-19 indicate a long-lasting circulation of specific immunoglobulins. Despite the relevance of the problem, research in this area remains poorly explored, especially regarding age-related differences. The prolonged circulation of elevated blood immunoglobulin numbers is possibly related to viral persistence and warrants further investigation of the problem.
All children were examined for pro-BNP, which is important in the diagnosis of heart failure. The average pro-BNP indices in the observed groups were within the normal range. However, when individual values were analyzed, it was found to be above the reference values (146.25 and 154.5, respectively) in 2 children from the first and second age groups — 5% (95% CI: -1.8 to 11.8). The clinical and prognostic significance of this laboratory phenomenon remains unexplored and is the subject of further research.
In the group of adolescents who had undergone COVID-19 and had cardiac complaints, a significant frequency of insufficient night-time BP reduction (non-dipper) was revealed, which is a predictor of arterial hypertension. The results of the study indicate a significant prevalence of various variants of heart rhythm disorders in the same group (35%). This finding is prognostically important, as this category of children has a predisposition to cardiovascular complications and target organ damage (17).
A detailed analysis of the results of 24-hour ECG monitoring revealed various variants of rhythm and conduction disorders in 35% of the children. In the group of children aged 4 to 7 years, QT prolongation was found in 2 children (5%). In the group aged 8 to 11 years, three children (7.5%) had abnormalities (2 with PQ prolongation, 1 with QT prolongation). In the adolescent group, heart rhythm disorders were detected in 8 children (20%): Three with ventricular extrasystole, 3 with QT prolongation, 1 with ventricular tachycardia, and 1 with AV-block 1 degree. Change in the QT interval (prolongation) is considered a risk marker for the development of dangerous ventricular arrhythmias, which requires additional examination. A fragment of the prospective examination of children who had undergone coronavirus infection showed long-term ECG changes in a comparative aspect.
5.1. Conclusions
The incidence of COVID-19 is more commonly reported in boys (64.75%), which is supported by several studies. This emphasizes the need to consider gender differences when designing preventive and treatment interventions. The majority of children experience a mild-to-moderate course of the disease, which aligns with data indicating a milder course of COVID-19 in children compared to adults. The most common symptoms in children are cough, tachycardia, and weakness, while dizziness and olfactory disorder are rare. The polymorphism of clinical manifestations requires a comprehensive approach to diagnosis. Significant cardiac changes, including increased fatigue, cardiac arrhythmia, and inadequate night-time BP decrease, have been identified in adolescents. This indicates a risk of cardiovascular complications and the need for long-term follow-up.
Heart rhythm abnormalities (PQ and QT interval changes, extrasystole, etc.) were found in 35% of children. These findings emphasize the importance of monitoring cardiac function in children after COVID-19. Elevated levels of IgG antibodies were observed in all age groups, indicating a persistent immune response. The duration and significance of this immunity remain the subject of further research. Mean values of pro-BNP are within normal limits in most cases, but elevated values have been found in selected children, requiring further study of their clinical significance.
Post-COVID-19 syndrome, including cardiovascular complications and fatigue, persists in some patients, emphasizing the need for further follow-up of these children. The findings require further investigation, especially with regard to cardiovascular and immune changes and their long-term consequences in children diagnosed with COVID-19.
5.2. Limitations
Limitations and shortcomings. The study population is limited to children and adolescents from the city of Aktobe, which restricts the generalizability of the findings to other regions and populations. Some age subgroups include an insufficient number of participants, potentially reducing the statistical significance and reliability of the results.
The analysis did not account for potential factors that may influence the long-term consequences of COVID-19, such as pre-existing health conditions, socioeconomic status, and access to medical care. The cross-sectional study design does not allow for the establishment of causal relationships between the observed symptoms, cardiovascular changes, and prior COVID-19 infection.
Although elevated IgG levels were detected, data on the durability of immunity remain limited, warranting further investigation. All of these limitations will be addressed in our future research through broader regional coverage, increased sample size, and the inclusion of additional variables for more comprehensive analysis.