1. Background
Cardiovascular disease, mainly caused by atherosclerosis, can inflict the greatest death in both developed and developing countries (1). Atherosclerosis is a chronic inflammation response towards cholesterol deposition on the arteries’ walls. Additionally, atherosclerosis plaque forming process is significantly affected by low-density lipoprotein (LDL) oxidation. Oxidized LDL (ox-LDL) uptake in macrophages leads to foam cell formation. The formed foam cells in turn result in secretion of pro-inflammation cytokines, such as tumor necrosis factor α (TNFα) and Interleukin-1 (IL-1) (2-4).
Lectin-like oxidized LDL receptor-1 (LOX-1) is one of the ox-LDL scavenger receptors in endothelial cells, which can contribute to atherogenesis through mediation of ox-LDL uptake. Moreover, ox-LDL causes endothelial cells dysfunction followed by an atherogenesis early phase inside the tunica intima (5-7). So far, atherosclerosis therapy has focused on anti-inflammation and anti-hyperlipidemia, which only inhibit atherosclerosis plaque progression, but not formation. Therefore, studies have to be conducted on atherosclerosis inhibition involving immune system as a promising preventive target. Peptide-based immunization is a choice to induce an immune response against atherosclerosis.
Recent studies have developed several vaccines against atherosclerosis, namely cholesteryl ester transfer protein (CETP) (8-11), IL-1 (12), and vascular endothelial growth factor receptor 2 (VEGFR2) (13), to induce protective immune response against atherosclerosis. Based on this approach, the present research aims to examine the potency of LOX-1 protein as an anti-atherosclerosis vaccine candidate. Although the role of LOX-1 in atherosclerosis is well known (2, 4-6), our study is the first to investigate the potency of LOX-1 as an anti-atherosclerosis vaccine candidate.
2. Objectives
Our study aims at investigation of the effect of LOX-1 protein on inhibiting NF-κB activity, eNOS expression, foam cells formation, and aorta wall’s thickness in atherogenic-diet Wistar rats (Rattus norvegicus).
3. Methods
3.1. Research Objects and Samples
After obtaining ethical approval from the ethics committee of faculty of medicine, Brawijaya university (No. 169/EC/KEPK-S1-FARM/03/2014), 28 healthy male Wistar-strain rats (Rattus novergicus) aging 6 - 8 weeks and weighing 120 - 160 g were enrolled into this study. The rats were divided into 7 groups, including a negative control group (normal diet with no vaccination), a positive control group (atherogenic diet with no vaccination), P1, P2, P3, P4 (atherogenic diet with 1, 10, 100, 1000 ng LOX-1 protein vaccination combined with alum as the adjuvant), and P5 (atherogenic diet with alum vaccination). The samples were selected using simple random sampling.
3.2. Atherosclerosis Induction
Atherosclerosis was induced by giving atherogenic diet every day for 56 days (14, 15). The normal and atherogenic diets used in this research were a modification of the American Institute of Nutrition-93M (AIN-93M) diet (16, 17), as shown in Table 1.
| Substrate | Weight (g/kg) | |
|---|---|---|
| Normal Diet | Atherogenic Diet | |
| Corn starch | 620 | 210 |
| Sucrose | 100 | 175 |
| Soybean oil | 40 | 50 |
| Gelatin | 65 | 50 |
| Casein | 80 | 128 |
| CMC | 50 | 51 |
| Mineral and vitamin | 5 pieces | 10 pieces |
| Fat | - | 105 |
| Coconut oil | - | 105 |
| Cholic acid | - | 3 |
| Cholesterol | - | 10 |
| Propylthiouracil | - | 2 |
Normal and Atherogenic Diet Compositions
3.3. LOX-1 Vaccination
The vaccines were prepared in a sterile condition by mixing 1, 10, 100, or 1000 ng of homodimer LOX-1 protein (Rat OLR1 Fc Tag) (Sino biological). Then, LOX-1 protein was dissolved in 100µL Phosphate Buffer Saline (PBS) and 100 μL alum as the adjuvant. Alum was provided by Professor Dr. Aulanni’am, DVM., DES, Faculty of Veterinary Medicine, Brawijaya University. The solution was prepared under sterile conditions. This mixture was subcutaneously injected on days 0, 21, and 35 (18-21).
3.4. Measurement of Anti-LOX-1
The rats were sacrificed using chloroform on the 57th day. Then, the aortas were isolated and blood samples were taken. The samples were centrifuged at 3000 rpm for 20 minutes to obtain the sera. After all, anti-LOX-1 IgG level was measured using ELISA method (Shanghai Crystal day Biotech Co, LTD).
3.5. Measurement of NF-kB and eNOS Expressions
Aorta slides were prepared using frozen section/fries coupe at -20°C and were cut into 3 - 5 µm thickness sections. The prepared slides were stained after methanol fixation for 5 minutes followed by three times washing with PBS and H2O2 addition. The blocking process was performed using Fetal Bovine Serum (FBS) containing 0.25% Triton X-100 for 1 hour. Then, anti-NF-kB (Sino Biological) and anti-eNOS (Source BioScience) antibodies were added and the slides were incubated overnight. On the next day, the slides were washed with PBS and were added to the secondary antibody for 1 hour. The streptavidin horse radish peroxidase (SA-HRP) was also added after slide washing followed by another incubation for 40 minutes. After washing, Diaminobenzidine (DAB) was added and the slides were incubated for 30 minutes. Counterstain was then performed with Mayer’s hematoxylin for 10 minutes. Thereafter, the slides were washed, dried, and covered using cover glasses. At each stage, the washing process was performed using PBS for three times. Finally, measurement of the cells with activation of NF-kB and eNOS was carried out using an optical microscope at 1000 × magnification.
3.6. Measurement of Foam Cells and Aorta Wall’s Thickness
Histopathology slides for measurement of foam cells and aorta wall’s thickness were obtained using the paraffin method. Then, the slides were stained using Hematoxylin-Eosin. Afterwards, the slides were examined at 400 × magnification for measurement of aorta wall’s thickness and at 1000 × magnification for computation of foam cells number via olympus photo slide BX51 Microscope.
3.7. Data Analysis
The data were analyzed using the SPSS statistical software, version 20 and P < 0.05 was considered to be statistically significant. After assessing the normality and homogeneity of the data, they were analyzed using one-way ANOVA and Tukey’s post-hoc test.
4. Results
4.1. Analysis of Foam Cells Formation and Aorta Wall’s Thickness
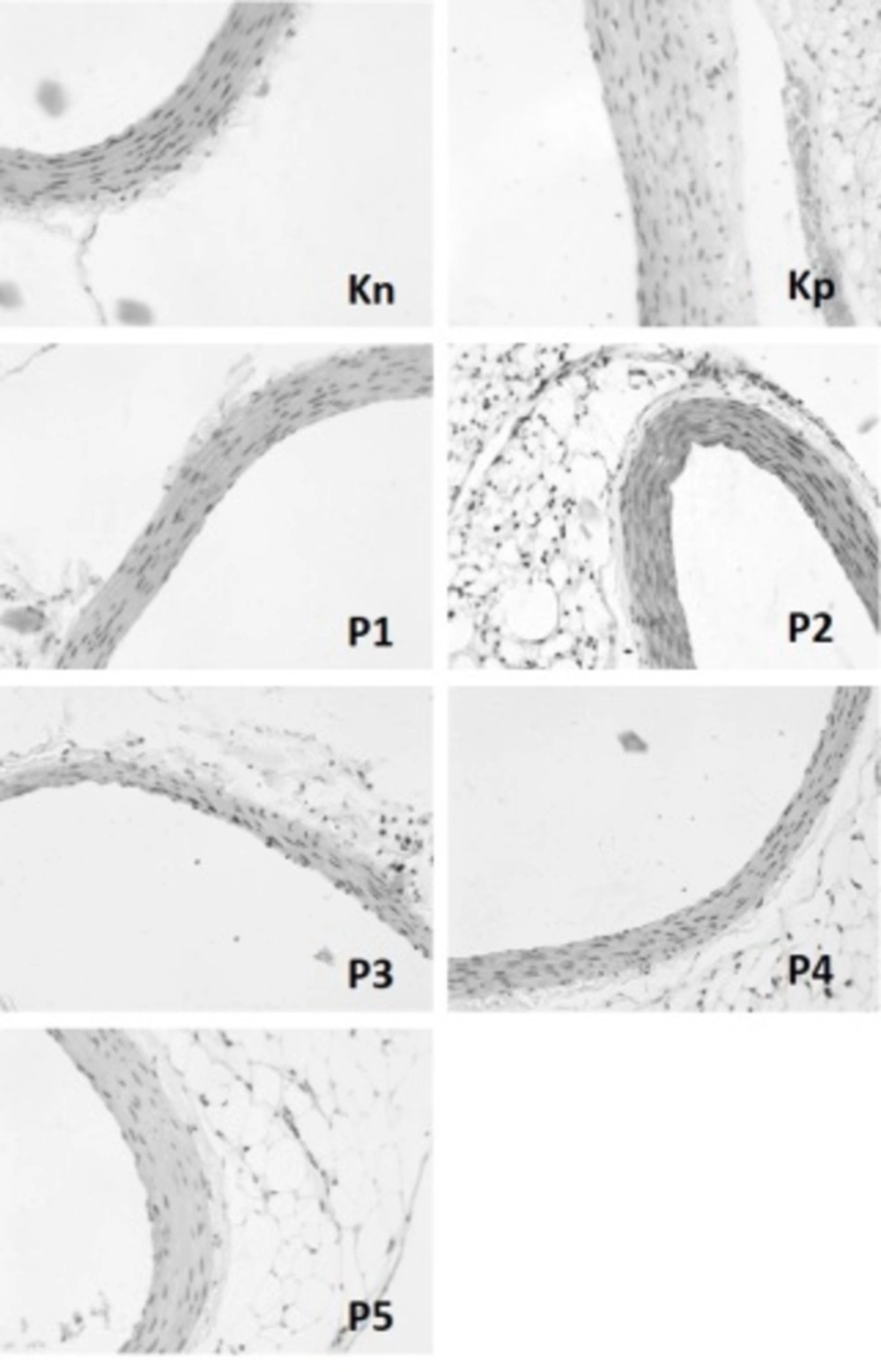
Generally, foam cells formation and aorta wall’s thickness are the major characteristics of atherosclerosis. Therefore, this study aimed to determine the foam cells number and aorta wall’s thickness using specific staining for each group. The mean of foam cells number and aorta wall’s thickness in each group have been presented in Table 2. The representative examples of aorta walls have also been depicted in Figure 1. The results revealed a significant difference between the non-treated group and all the vaccinated groups with atherogenic diet regarding the number of foam cells. Nonetheless, the treatment group that received 1000 ng LOX-1+alum was similar to the normal diet group in this regard (P = 0.055). This indicated that LOX-1 vaccination inhibited the formation of foam cells. A similar trend was also detected with respect to aorta wall’s thickness. Accordingly, LOX-1 vaccination significantly inhibited aorta wall’s thickening. In other words, all the treated groups showed thinner aorta walls compared to the non-treated group.
| Groups | Foam Cells Number | P Value (vs. Kp) | Aorta Wall’s Thickness (µm) | P Value (vs. Kp) |
|---|---|---|---|---|
| Normal diet without vaccination (Kn) | 0.78 (0.30) | 0.001 | 1.83 (0.10) | 0.001 |
| Atherogenic diet without vaccination (Kp) | 7.15 (0.13) | 3.55 (1.18) | ||
| Atherogenic diet with 1 ng LOX-1 vaccination (P1) | 3.25 (0.72) | 0.001 | 1.81 (0.04) | 0.009 |
| Atherogenic diet with 10 ng LOX-1 vaccination (P2) | 2.73 (0.56) | 0.001 | 1.77 (0.61) | 0.007 |
| Atherogenic diet with 100 ng LOX-1 vaccination (P3) | 2.73 (0.87) | 0.001 | 1.63 (90.17) | 0.003 |
| Atherogenic diet with 1000 ng LOX-1 vaccination (P4) | 2.25 (0.37) | 0.001 | 1.60 (0.19) | 0.002 |
| Atherogenic diet with alum vaccination (P5) | 3.23 (0.42) | 0.001 | 1.84 (0.21) | 0.017 |
Foam Cells Number and Aorta Wall’s Thickness [Mean (SD)]
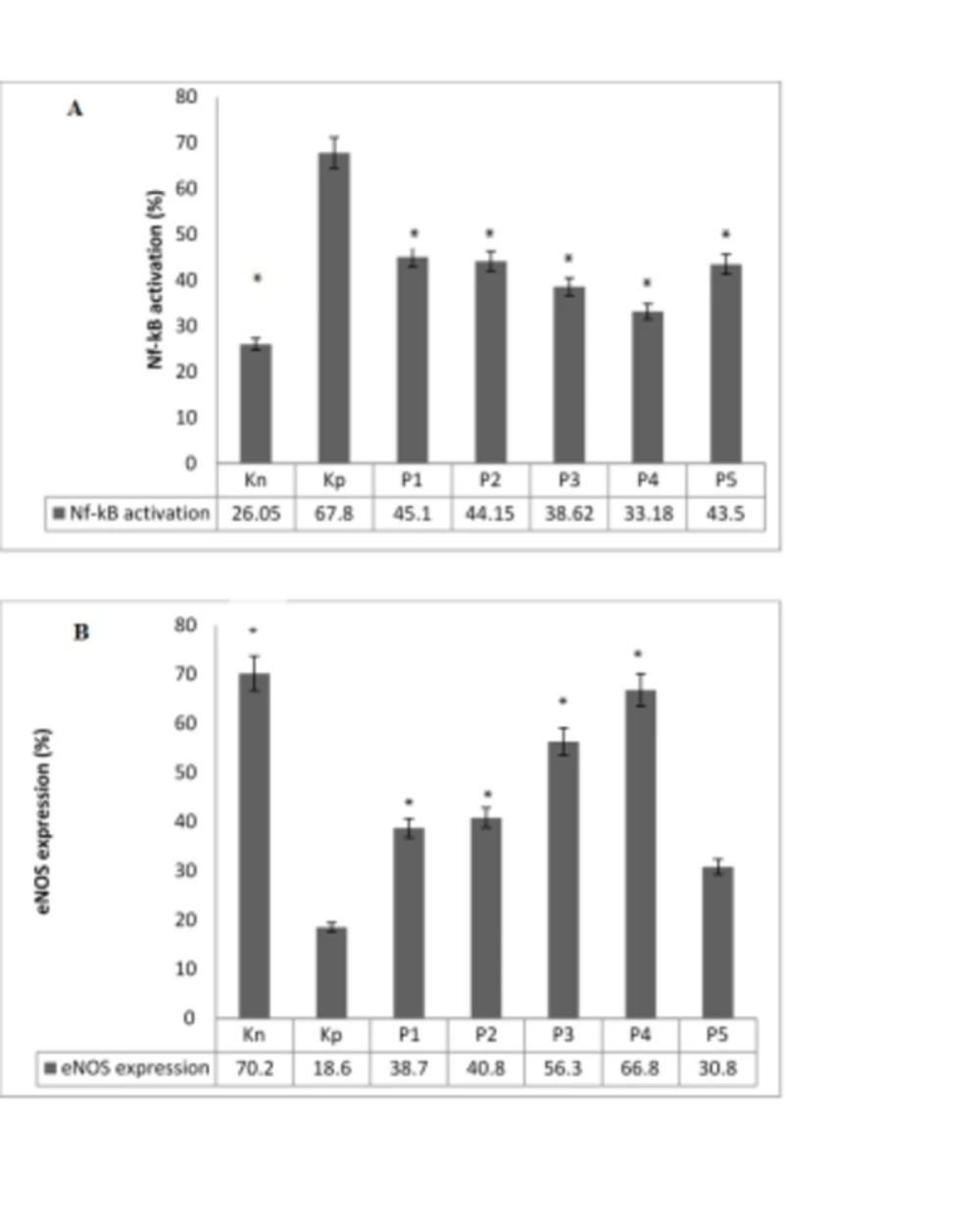
4.2. LOX-1 Administration Inhibited NF-κB Activation and Stabilized eNOS Expression in Endothelial Cells
In general, NF-κB activation and reduction of eNOS expression show endothelial cells dysfunction (22). The current study also assessed NF-κB activation and eNOS expression in aortic sections from different groups of rats. The results have been presented in Figure 2. Accordingly, the NF-κB activation significantly reduced in the vaccinated groups compared to the non-vaccinated group. On the other hand, the LOX-1 vaccination normalized the expression of eNOS. Interestingly, the group only treated by alum showed a reduction in eNOS expression compared to the LOX-1 vaccination groups.
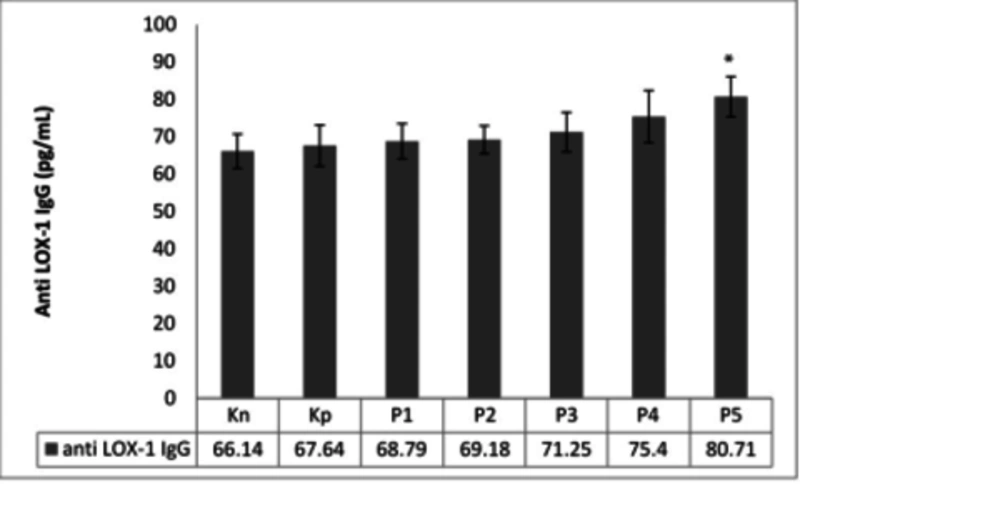
4.3. The Effect of LOX-1 Administration on Anti-LOX-1 Ig-G Level
LOX-1 administration was expected to raise IgG anti-LOX-1 production that can inhibit the interaction between ox-LDL and LOX-1. Although LOX-1 is an endogenous protein, LOX-1 administration combined with the alum as the adjuvant was presumed to increase the anti-LOX-1 level. However, administration of LOX-1 in various concentrations (1, 10, 100, and 1000 ng) in combination with alum failed to raise the serum level of IgG anti-LOX-1 (Figure 3).
5. Discussion
Atherogenic diet promotes atherosclerosis by increasing LDL, changing it into its oxidized form, ox-LDL. ox-LDL uptake, in turn, leads to activation of NF-κB (6, 23). A previous study showed that NF-κB activation increased as an inflammatory response that stimulated endothelial dysfunction in an atherosclerotic rat model (23). In the present study also, vaccination using LOX-1 protein significantly prevented endothelial dysfunction, proven by the inhibition of NF-κB activation and stabilization of eNOS expression.
Similar results were also obtained in the previous researches. For instance, in vivo given anti-LOX-1 antibody restored. NO-mediated coronary arteriolar dilatation in ApoE-/- mice (24). Similar to the in vivo model, an in vitro experiment showed that LOX-1 and Membrane Type 1 Matrix Metalloproteinase (MT-1-MMP) complex contributed to downregulation of reactive oxygen species (ROS) formation and eNOS expression (25). Moreover, the studies conducted in patients with stable coronary artery disease (CAD) or acute coronary syndrome (ACS) indicated that high-density lipoprotein (HDL) could trigger LOX-1 expression and consequently increased endothelial Protein Kinase C β2 (PKCβ2) activation, which led to eNOS activation (26). In short, LOX-1 played a central role in regulating. NO-mediated vascular reactivity.Activation of NF-κB induced increase of vascular endothelial adhesion molecule (VCAM-1), intracellular endothelial adhesion molecule (ICAM-1), and monocyte chemoattractant protein (MCP-1) expressions (25, 26). Furthermore, increase of the adhesion molecule led to attraction of monocytes followed by lipid accumulation that resulted in foam cells formation. Indeed, inhibition of NF-κB activation repressed the formation of foam cells (27, 28). Our findings demonstrated that inhibition of NF-κB activation successfully blocked the formation of foam cells. Decrease in the number of foam cells, in turn, affected the reduction of aorta wall’s thickness in the vaccinated rats. Hence, vaccination of LOX-1 protein protected atherosclerosis by inhibiting foam cells formation and aorta wall’s thickening.
Although the importance of LOX-1 protein in development of atherosclerosis has been studied extensively (3, 6, 26), our study was the first to demonstrate the potential inhibition of atherosclerosis through LOX-1 vaccination. In this study, LOX-1 protein combined with alum was used to induce anti-LOX-1 IgG production. Besides, the LOX-1 vaccination was given in 1 - 1000 ng dosages, and alum was used as the adjuvant. Generally, alum acts as a delivery system to promote antigen uptake into immune cells. It is one of the most useful adjuvants with multiple pathways to stimulate the immune system. Moreover, alum plays a role as a depot system by allowing retention of antigen in the injection site. In this way, the antigen is released slowly and the recruited antigen presenting cells can interact extensively (29-31). Alum also has anti-atherogenic properties. Therefore, this adjuvant may be suitable for developing anti-atherosclerosis vaccines. However, the hypercholesterolemia condition may shift the immune response induced by alum towards activation of Tregs. Moreover, individuals with hypercholesterolemia may develop tolerance against the antigen given through vaccination (20). Although the antibody against LOX-1 was not produced, the results indicated that our vaccination inhibited atherosclerosis progression. In the same line, apo-B peptide vaccine inhibited atherosclerosis without activating apo-B specific antibody, suggesting that the atheroprotection effect was modulated through cellular immune response (18). The CD8+T cell has been shown to play a role in atheroprotection, as well (32-34). Nevertheless, the role of IgG in atherosclerosis is still unclear. IgG had an anti-atherosclerosis function through its neutralizing action. However, it induced activation of macrophages, thereby promoting atherogenesis (35). Another possibility is that LOX-1 acts as a soluble receptor for ox-LDL. Therefore, ox-LDL uptake is inhibited that leads to inhibition of atherosclerosis progression. Yet, the pathophysiological role of soluble LOX-1 remains unclear. Neither signaling nor scavenging activity of this soluble receptor has been identified (36).
5.1. Conclusion
LOX-1 vaccination significantly decreased the formation of foam cells and thickening of the aorta’s wall compared to the non-vaccinated atherogenic-diet Wistar rats. LOX-1 vaccination also inhibited the increase of NF-κB activity and stabilized the expression of eNOS in endothelial cells of atherogenic-diet Wistar rats. Thus, LOX-1 protein vaccination might have atheroprotection effects through modulation of cellular immune response. In addition, it might act as a soluble receptor inhibiting ox-LDL uptake in endothelial cells.