1. Background
It is asserted that combining low-intensity resistance training with blood flow restriction increases muscle volume similar or even more than high-intensity traditional resistance training. There has been gained an intense interest in understanding the responsible mechanisms for this muscular structural adaptation (1, 2). Previous studies on the effect of BFR training on strength, hypertrophy, and muscular endurance have now raised the question of whether exercise is generally safe. The safety of BFR training as a result of these exercises emphasizes the response of the cardiovascular system (both central and peripheral), muscle damage, oxidative stress, and nerve conduction compared to regular training (3, 4). Recently, researchers have not confirmed the evidence that BFR, in combination with low-intensity resistance training, increases the muscle damage. According to the literature, if this training pattern is followed, athletes will experience the least damage, or even no muscle damage will occur. Besides, it causes no long-term decrease in muscle function, muscle inflammation, muscle bruising, and no increase in blood indicators of muscle damage will happen (1).
Recent studies have shown that increased protein synthesis after a BFR training session is associated with post-translational regulation of the AKT/mTOR pathway (1). Also, a reduction in genes associated with FOXO3a, atrogen, and Murf-1 proteolysis. Moreover, reduced levels of negative regulatory factors for muscle mass and myostatin in 8 and 48 hours after short-term training in combination with BFR are also reported (5). Even a study has reported an increase in muscle hypertrophy and muscle strength after 20 minute treadmill aerobic running exercise at 45% of heart rate reserve intensity in the elderly (64 ± 1 year) (6). A study that aimed to investigate the response of angiogenesis to blood flow restriction (BFR) training, the BFR effect is observed by increasing the expression of genes affecting the angiogenesis process in skeletal muscle tissue, while this training pattern does not affect the serum and VEGF protein levels (7). It is proved that the combination of intense resistance training with vibration and interval application of BFR on genes involved in the process of angiogenesis and mitochondrial biogenesis activate angiogenesis and metabolic program, while typically activating in response to endurance training, angiogenesis, and mitochondrial biogenesis (8). Some researchers reported an increase in skeletal muscle IL-6 following 17 sessions of resistance training in diabetic rats (9), while others reported a decrease in serum IL-6 following 8 weeks of resistance training in inactive middle-aged men (10).
A rapid increase in serum levels of MMP-9 at the end of exercise has been observed in studies that have used eccentric activity and progressive step-by-step testing to the point of exhaustion (11, 12). New research has reported changes in the levels of conduction, resistance, and capillaries in the vascular network in response to low-intensity resistance training with BFR, and adaptation occurs in different positions of the vascular network at different time limits. According to recent hypotheses, early changes occur at the end of the vascular network (13). This is compounded by the increased blood pressure, the reaction that occurs before structural changes in papillary vascular capacity, as well as the rapid response of angiogenesis to resistance training with BFR (14).
It worth noting that IL6 and MMP9 factors are activated during and after BFR training and angiogenesis response in the arteries stimulated. However, the response of angiogenesis to the low-intensity eccentric resistance model with BFR and high-intensity resistance training has not yet been fully reviewed (7). Examining and determining the association between these factors after doing exercise with BFR would be helpful in understanding the effective mechanisms of BFR training on the vascular network.
2. Objectives
Therefore, the current study aimed to determine the effect of two eccentric resistance training methods with and without the restriction of blood flow on serum IL6 and MMP9 of active young men.
3. Methods
This is a semi-experimental study which was performed in a laboratory manner. The subjects included 16 healthy males who did not have a history of regular resistance training. Participants were divided into two groups, each with eight members using the Internet randomization program and randomized block methods. The order of entry of the subjects and their exercise program was determined using the https://www.sealedenvelope.com/simple-randomiser/v1/lists. The research protocol was performed for 4 weeks in which the participants performed two resistance training methods with and without blood flow limitation with at least one week apart. In the first session, participants first completed personal and health questionnaires that contained personal information, written informed consent, medical history assessment, lifestyle assessment, and physical activity evaluation. The researcher then provided them with necessary explanations about different stages of the research and how it will be performed. Demographic characteristics and hemodynamic variables of the subjects were measured before performing the test (Table 1).
| Variables | Mean ± SD | Minimum | Maximum |
|---|---|---|---|
| Age, y | 27.8 ± 2.85 | 23 | 35 |
| Weight, kg | 79.4 ± 12.4 | 55.5 | 97.1 |
| Height | 176.1 ± 6.03 | 165.5 | 187.5 |
| Body mass index, kg/m2 | 25.5 ± 3.7 | 19.5 | 33.8 |
| Resting heart rate, beats/min | 73.06 ± 9.28 | 52 | 87 |
| Systolic blood pressure, mmHg | 119.1 ± 6.44 | 107 | 127 |
| Diastolic blood pressure, mmHg | 77.5 ± 7.66 | 68 | 95 |
3.1. Warm-Up and Determining MVC
Initially, the subjects were warmed up on a standard carburetor bike (Monark Model 839, made in France) with a rate of 60 - 50 rpm (rpm), which is about 75 watts and burned about 17 kcal and the MET was 2/3 to 2/5. They did it for 5 minutes (15). To ensure the intensity of the heartbeat, subjects were assessed with the Polar T31 (Polar T31) pacemaker and reached about 100 - 140 beats per minute. To determine the MVC, after placing participants on the isokinetic device (Biodex, USA), the anatomical position was coordinated with the device’s connections, then the subject’s foot was placed at a 70-degree angle (relative to the zero-degree source in full knee opening). Subjects were then asked to apply the maximum amount of force possible to the quadriceps muscle and forward, the contraction time was 5 seconds, and the protocol was performed 3 times. The maximum amount of force that the subject could apply in an instant was considered as MVC (2).
3.2. The Eccentric Resistance Training Program
Eccentric resistant training (ECC RET) is similar to the protocol used by Nielsen et al. (16), that used an isokinetic device applied in previous research to separate the eccentric part of muscle contraction and load control applied in the eccentric and concentric phase of the movement.
The training protocol involved performing an eccentric contraction of the quadriceps muscles with 20% - 30% MVC and limited blood flow to the limit of exhaustion for about 3 - 5 periods and with 30, 15, 15, 15, and 15 repetitions, respectively, and 45 to 60 seconds of rest. The training protocol involved 5 sets (30, 15, 15, 15 and 15 repetition) quadriceps muscle contraction at the intensity of 20% - 30% maximum voluntary contraction and about 50 percent blood flow restriction to the exhaustion point, and 45 to 60 second rest interval in low-intensity resistance group, whereas high-intensity eccentric resistance exercise performed same number quadriceps muscle contraction interval at 70% - 80% maximum voluntary contraction intensity without blood flow restriction. The training sessions were held using the US-isokinetic biodex device (16).
3.3. Limiting Blood Flow
Blood flow restriction was performed using a 13 cm wide device (manufactured by the German company Richter) with the ability to apply pressure up to 700 mm Hg. Before performing the exercise, 90 - 100 mm Hg of the device was inflated in the eccentric resistance training group with the BFR, while the device tape was located in the upper thigh, and this level of pressure was maintained during the training and rest times between training sets. Immediately after the end of the last set, the blood flow restriction was removed, and then cooling down activities were performed for 5 minutes, which included stretching and flexibility.
3.4. Sampling
Blood sampling was performed in two stages with an interval of eight days. At each stage twice (both before and after the training) 5 cc of blood was taken from the anterior vein and poured into tubes containing sodium citrate to separate plasma and anticoagulant tubes from the serum for further analyses. It was sent to the laboratory and kept at a temperature of -20°C.
3.5. Measuring Serum IL6 and MMP9
Serum IL6 and MMP9 were measured using kits (Bioassay Laboratory Technology, Crystal day, China, Cat, No: E0090Hn) and (Bioassay Laboratory Technology, Crystal day, China, Cat, no.: E0936Hn) and ELISA method.
3.6. Data Analysis Method
Initially, the Shapiro test was used to test the normality of data distribution. To analyze and compare pre-test and post-test factors measured in two training groups, the paired t-test was used. To compare changes before and after the training program in each group and also to compare changes between two groups analysis of variance with repeated measure was employed. Data were analyzed in SPSS version 22. A P value of < 0.05 was considered as statistically significant.
4. Results
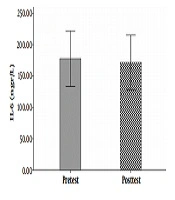
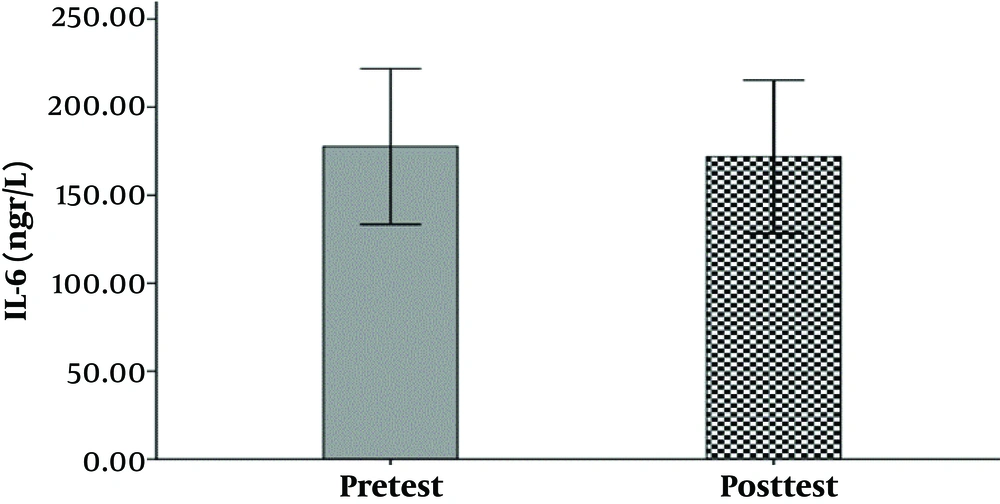
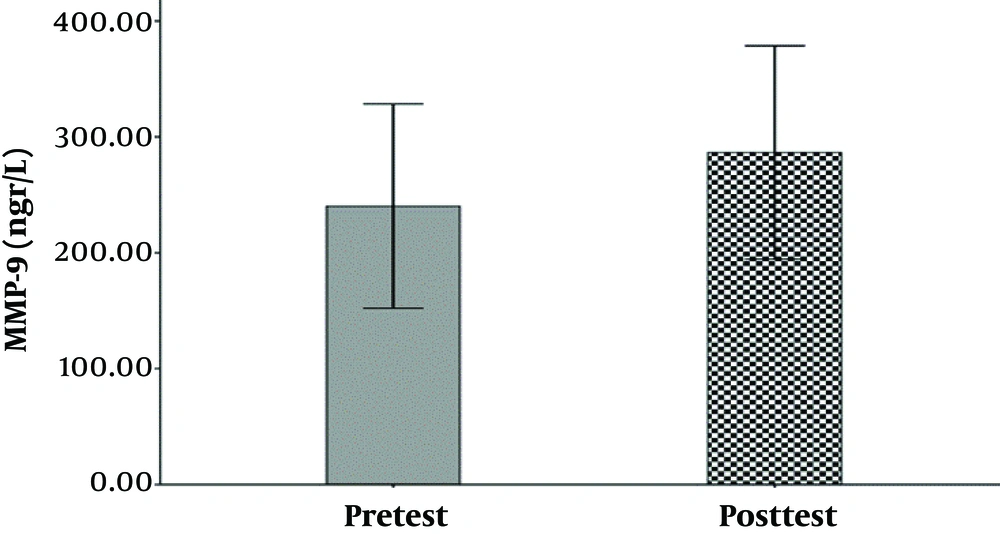
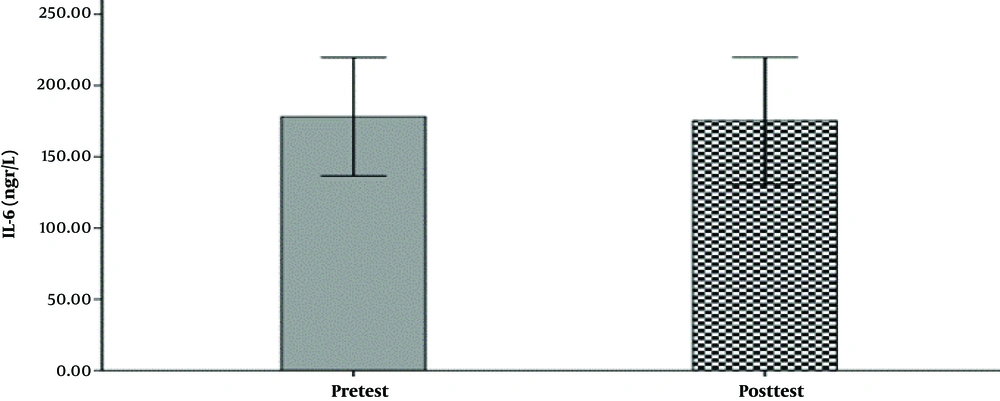
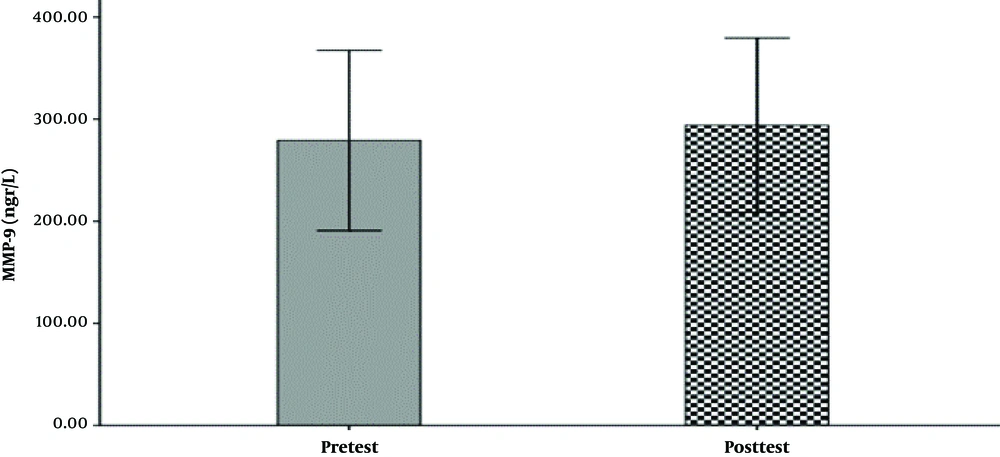
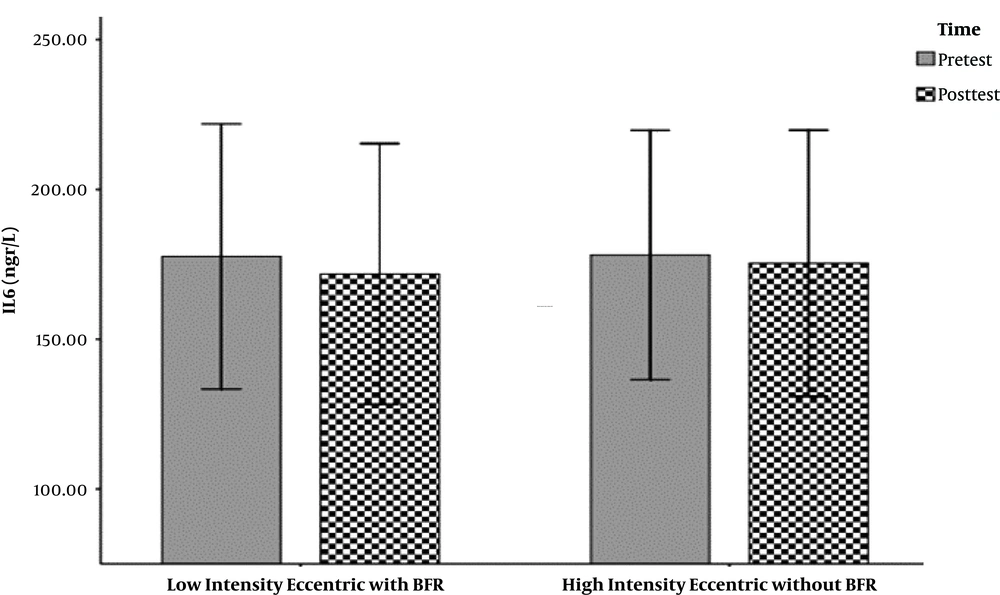
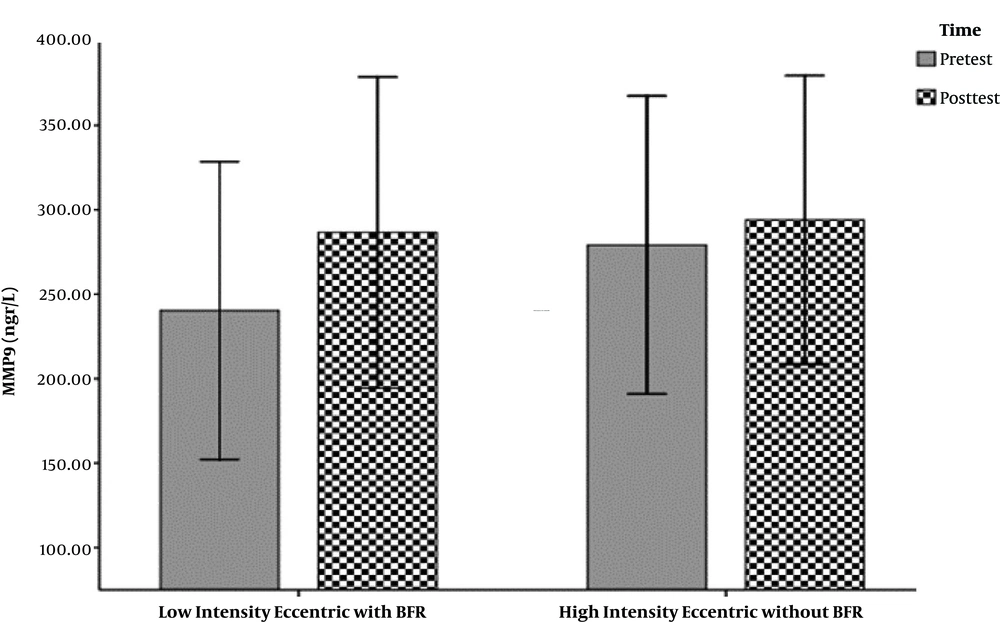
The hemodynamic variables of the subjects, including heart rate, systolic and diastolic blood pressure, and their demographic characteristics (age, height, weight, and body mass index) are summarized in Table 1. The mean and standard deviation of serum levels of IL-6, MMP-9, and the results of the Shapirovilek test are provided in Table 2. The results of the pair sample t-test showed a low-intensity resistance training session with blood flow restrictions on serum IL-6 (t (15) = 1.189 and P = 0.253) and MMP-9 (t (15) = -0.832 and P = 0.419).
The results of the pair sample t-test showed a low-intensity resistance:
training session with blood flow restrictions did not have a significant effect on serum IL-6 (t (15) = 1.19 and P = 0.25) and MMP-9 (t (15) = -0.83 and P = 0.419) in active young men. The results of the analysis of variance test with a repeated measure of 2.2 to compare serum IL-6 levels in two types of exercise and two times showed no significant interaction between time and type of exercise (F (1, 15) = 0.107 and P = 0.748). Also, the main effect of time (F (1, 15) = 0.656 and P = 0.431) and the main effect of the exercise type (F (1,15) = 0.006 and P = 0.937) were not significant. The results of the analysis of variance test with repeated measurements of 2.2 to compare serum levels of MMP-9 in two types of exercise and two times indicated no significant interaction between time and type of exercise (F (1, 15) = 0.290 and P = 0.598). Also, the main effect of time (F (1, 15) = 1.041 and P = 0.324) and the main effect of the type of exercise (F (1, 15) = 0.827 and P = 0.378) were not significant (Figure 1-6).
| Parameter, ng/L | Type of Exercise | |||
|---|---|---|---|---|
| Low Pressure Eccentric with BFR | High Pressure Eccentric Without BFR | |||
| Pre test | Post test | Pre test | Post test | |
| IL-6 | 177.63 ± 83.04 | 171.77 ± 81.63 | 178.11 ± 78.02 | 175.35 ± 83.32 |
| P value Shapiro | 0.117 | 0.065 | 0.085 | 0.094 |
| MMP-9 | 240.28 ± 165.42 | 286.53 ± 172.89 | 279.18 ± 165.54 | 294.12 ± 160.31 |
| P value Shapiro | 0.067 | 0.139 | 0.121 | 0.219 |
aValues are expressed as mean ± SD.
5. Discussion
According to the results, during and after BFR training, many blood factors are activated and angiogenesis is stimulated in the arteries. The current study aimed to determine the effect of two eccentric resistance training methods with and without blood flow restriction on serum IL6 and MMP9 levels of active young men.
The results showed that a low-intensity resistance training session with blood flow restriction and also a high-intensity resistance training session without blood flow limitation did not have a significant effect on serum IL-6 serum levels. This result is consistent with the results of Larkin et al. (7) and Item et al. (8). And it is inconsistent with Melanouri and Mahdavi (9).
High-intensity training can increase myoglobin, lipid peroxidase (LP), creatine kinase (CK), and cytokines (IL-6, TGF-β, TNF-α) in the short-term. Takarda et al. (17) investigated the effects of BFR in combination with 20% 1RM resistance training on CK, LP, and IL-6 concentrations. After implementing the protocol for 90 minutes the IL-6 was increased and no change was observed in other variables (17), which is inconsistent with the results of the current study. This inconsistency can be attributed to the type of performed protocol and the duration of the resistance exercise. Melanouri and Mahdavi (9) reported an increase in skeletal muscle IL-6 following 17 sessions of resistance training in diabetic rats and Mir et al. (10) reported a decrease in serum IL-6 followed by 8 weeks of resistance training in inactive middle-aged men.
In the study of Melanouri and Mahdavi (9) the reason for increased interleukin and the lack of increase in this factor in the current study can be attributed to the characteristics of participants and the nature of the training course in which diabetic rats participated and the duration of the training course was 17 sessions, but in the present study the participants were active males and the duration of the training was one session. Also, decreased levels of serum IL-6 in the study of Mir et al. (10) and its stability in the current study can be attributed to the characteristics of participants, the length of the training period, and the level of training, in which in the current study no active middle-aged males participated. The resistance training program was implemented for 8 weeks, but in our study, participants were active young man and their training program consisted of one resistance training session.
Most studies have used blood flow restriction during exercise. According to the literature, the type of cuff used may affect the results. In some studies, it has been used as an elastic cuff and in others as a non-elastic one. Rossow et al. (18) investigated cardiovascular responses in the presence of restricted blood flow in resistance sports and reported that the type of limiting device had an effect on cardiovascular responses and should, therefore, be considered when designing studies. Yasuda et al. (19) also showed that resistance training with an elastic band and blood pressure restriction did not have a negative effect on adult arterial stiffness and improved muscle cross-section and maximum strength. Also, these studies showed the effect of blood flow restriction on increasing the expression of genes affecting the process of angiogenesis in skeletal muscle tissue, while reported no effect on serum and protein levels of VEGF (8).
Another study found an increase in MMP-9 marker levels in venous arteries during a training session, however, no MMP-9 analysis was observed in the studied muscles (20). This increase was also observed at the end of eccentric sports activities and progressive step testing to the point of exhaustion (11, 12), while in the present study a low-intensity resistance training session with high blood flow and high intensity on MMP-9 serum levels young active men was not different. Which is not consistent with previous studies.
Numerous studies have examined skeletal muscle as a source of increased circulating MMP-9 release. In some studies, only intravenous MMP-9 concentration is measured, and the increase observed in MMP-9 concentration reflects its release by skeletal muscle exercising. These data suggest that MMP-9 is less likely to increase circulation than other sources (other than skeletal muscle) (11). MMP-9 is a diverse protease with a significant underlying panel, and it has been shown that different cell types can express and secrete MMp-9. On the other hand, MMP-9 has little basic expression in most tissues (21). Laboratory studies have reported an increase in MMP-9 activation of post-exercise skeletal muscle (20). MMP-9 also appears to be activated in post-workout muscle tendons (22), which explains why the MMP-9 serum levels do not differ in two intensities in the present study. The most basic biological and basic function of MMP-9 is the breakdown of extracellular matrix proteins. Total levels of MMP-9 (pro-MMP-9 and active mmp-9) in skeletal muscle following exercise are significantly faster than pre-workout values. After exercise, it increases and stays at a high level for up to 120 minutes. Therefore, since MMP-9 is released by active muscles, MMP-9 is a potential candidate and an increase in MMP-9 circulatory levels following severe muscle damage is reported (20).
So far, it has been shown that the age range of the participants was ranging from 20 to over 50 years old (23) and these subjects with different physical fitness levels as some were untrained (24), recreationally active (25) and elites athletes (17) in Practices have been recruited in pervious research. The effects of differences between men and women in BFR require further research and more study has been done on men.
However, it is not clear exactly how much blood flow restriction is enough to stimulate training response as in pervious studies is between 100 mmHg and 300 mmHg pressure in pneumatic cuff were applied to induced restricted or incomplete blockage of working muscle blood flow. Some studies have performed complete repetition at any time, while in others, the activity has been executed up to the fatigue (17, 24, 26). In present study we applied 10 mm Hg above mean systolic blood pressure to induced about 50% blood flow restriction.
Takano et al. (27) reported a significant increase in their indices at low pressures of 50 mmHg. Takano et al. (27) observed a 30% reduction in arterial blood flow following a 160 to 180 mmHg blood flow restriction with a 33 mm cuff width. By increasing the cuff width (pressure relief tool), the required pressure to restrict blood flow was reduced (3). In various studies, the width of the cuff varied from 5 to 20.5 cm. In the present study, which a 13 cm wide pneumatic band was used, the amount of pressure to apply the restriction of blood flow was 100 - 90 mmHg. Decreased blood flow reduced intramuscular oxygen and metabolic clearance, which led to the accumulation of lactic acid and the onset of fatigue, which is determined by a decrease in the tone and number of repetitions of a single resistance activity (27, 28).
Overall, given that in the current study intense exercise, especially in high intensity (i.e. eccentric resistance training with about 80% - 70% MVC), did not induce a significant effect on serum levels of IL-6 and MMP-9 of active males, it can be argued that more intense exercise leads to the release and production of countless free radicals that in turn results in elimination or inactivation of nitric oxide (NO) produced, subsequently disrupted these process as nitric oxide is an important and effective factor in angiogenesis. One of the limitations of the present study is the short duration of eccentric resistance exercise executed by active male volunteers, which could be cleared in further studies.
5.1. Conclusions
Overall, the results of the present study showed that a low-intensity resistance exercise session with blood flow restriction, such as high-intensity resistance exercise session without blood flow restriction, can increase the serum levels of IL-6, which was not statistically significant. While decreased level of MMP-9 was not statistically significant among active males. These results can be attributed to the duration and intensity as well as the type of exercise protocol, number of times and repetition of courses, and physical fitness levels of the participants. Further studies are needed to identify the exact volume and modality of exercise required to stimulate optimum exercise-induced angiogenesis response.