1. Background
The overgrowth of aquatic plants causes problems for the function of water treatment plants. However, the aquatic plants play important roles in the maintenance of the ecological balance in lakes, ponds, and rivers. Algae are primary plants without factual leaves, stems, and roots (1). They classify into green algae, cyanobacteria, red algae, yellow-green algae, Chrysophyceae, etc. Cyanobacteria constitute the most important group of algae. They can cause many problems such as toxin release, which can be detrimental to the quality of drinking water and lead to diseases in humans and animals. Cyanobacteria can form colonies and grow quickly in large masses (2, 3). Cylindrospermopsis raciborskii belongs to Nostocaceae family and Oscillatoriales order from cyanobacteria. Echinite and growing cells make them distinguishable. Humans poisoning and animal death due to C. raciborskii bloom are reported in different countries such as the USA, Iceland, the Netherlands, and Australia (4, 5). Physical and chemical variations due to seasonal changes can cause an algal bloom. Algal overgrowth can cause operational problems in water systems such as changing taste, odor and color of the water, causing toxicity, sludge accumulation, and corrosion, as well as trihalomethanes (THMs) release. Consequently, it probably affects the ecological systems and causes the domination of cyanobacteria. Zooplanktons do not use cyanobacteria as food sources. They consume algae that compete with cyanobacteria. In this process, necessary nutrients are not consumed and they improve cyanobacteria growth (6, 7). Nutrients such as nitrogen and phosphorus are necessary for the growth of cyanobacteria. One of the major pollutants found in aquatic environments is phosphorus. The average amount of phosphorus in water resources is < 1 mg/L; exceeding the amounts permitted in water causes a serious threat to the environment, animals, and aquatic life (8-10). Phosphorus is a limiting factor in lakes. A little rise in the content of this nutrient influences the toxin production, since it increases the growth of the alga. According to previous reports, reducing phosphorus content results in microcystin and anatoxin reduction (11-13). Produced toxins are structurally different with various complications from liver cancer to neurotoxicity (14). Some of these toxins accumulate biologically in the food chain and can enter human and animal life cycle. Recent studies to control and prevent diseases by the American institutions show that harmful algal bloom (HAB) is transferred by air through wind and wave motion. Humans are likely to inhale HAB even in a far distance of polluted waters (15). Phosphorus content is an influential factor in algal growth. Hence, decline in phosphate during the growth season is a limiting factor for algal growth.
2. Objectives
The current study aimed at investigating the influence of different phosphate contents on growth of C. raciborskii.
3. Methods
A stock of C. raciborskii was provided which tested with a Neubauer slide and optical microscope (Nikon) at a 100× magnification. All experiments were performed at three phosphorus concentration and one control group was cultured in Zehnder 8 (Z8) (using Z8 without a nitrogen source) and Blue-Green 11 (BG11) media. First, the alga was purified and the experiments were performed under laboratory conditions (temperature 25°C ± 1 and light intensity of 2200 lux). Thalli were counted daily for 12 days. After 72 hours, a wide variety of phosphorus colors from green (in Z8) to yellow (in BG11) was observed. Finally, sample counting was performed daily in triplicate for every sample by Neubauer chambers. The rates of cell division one day (G) and specific growth (µ) were calculated using Equations 1 and 2 (16):
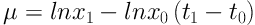
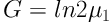
x0: The average number of cells at t0
x1: The average number of cells at t1
μ: The rate of specific growth (daily)
G: Necessary time for per cell division (day)
To analyze the data, an accidental plan was used in MSTAT-C software on the days 2, 4, 6, 8, 10, and 12 in three media containing phosphorus 150, 300, and 600 µg/L. To determine the effect of phosphorus and compare the average values of parameters, one-way analysis of variance and Duncan post hoc test (P value < 0.05) were independently and simultaneously used for each phosphorus concentration. Furthermore, SPSS and Excel were applied to analyze the data.
4. Results and Discussion
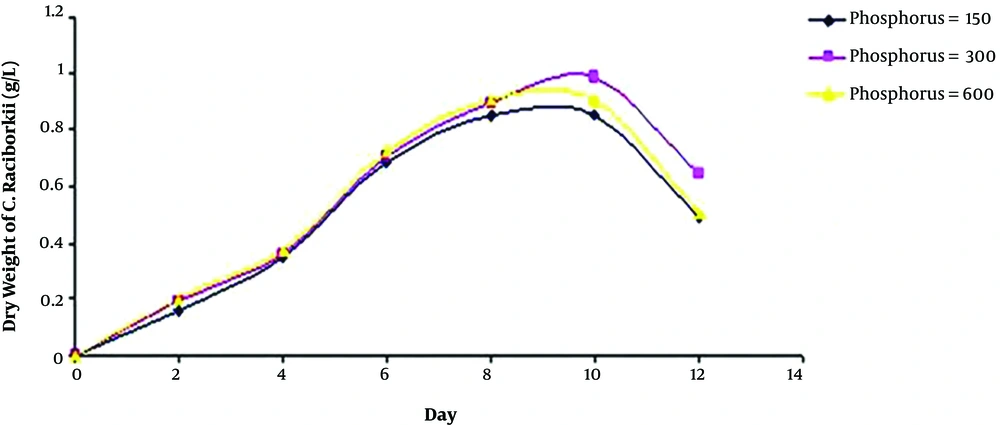
The dried mass of C. raciborskii on different days of cultivation showed no significant difference in the produced biomass among the three phosphorus concentration groups by the day 8 of cultivation, according to One-way ANOVA and Duncan test results.
Nevertheless, it increased significantly on the day 10 at 300 µg/L of phosphorus, when compared to those of the two other groups. According to Figure 1, there was an increasing trend in growth from the beginning to the day 8 in all the three groups. However, on the day 10, it was close to the death phase.
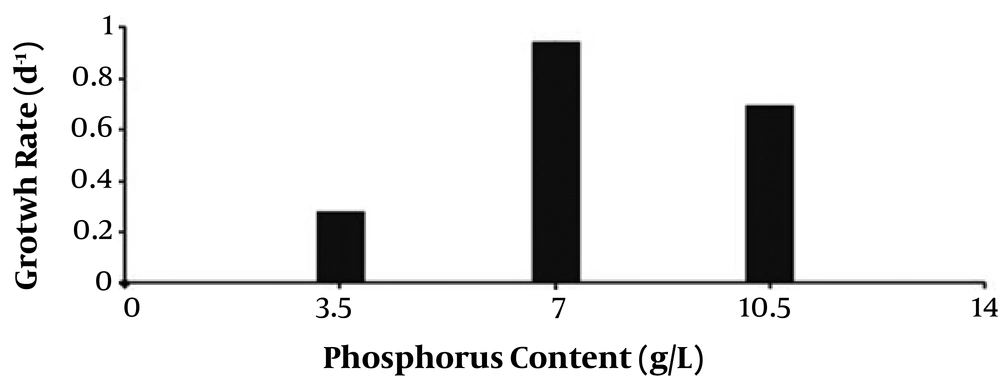
According to Figure 2, the growth was much higher at a phosphorus concentration of 7 g/L compared with those of the triple contents of phosphorus and the control group. No significant effect of treatment was observed in growth rate of C. raciborskii (P > 0.05) exposed to different contents of phosphorus. The highest specific growth rate (µ: 0.9 daily) belonged to the group treated with 7 g/L phosphorus (Figure 2).
The influence of different phosphorus concentrations on the growth of C. raciborskii was studied by Shafik et al. and it was found that under different phosphorus concentrations, the highest recorded growth rates were 0.8 - 1 daily that was in agreement with those of the current study. Shorter day length decreased growth rates of cyanobacteria and diatoms, and this decrease was greater in cyanobacteria (17); this finding also consistent with that of the current study. Previous investigations demonstrated the increased cyanobacteria growth during long days of the year (18). The results of the current study showed that C. raciborskii growth was compatible with the phosphorus concentration changes. Cells used for cultivation in different groups were picked from the growth phase of stock cells. These cells continued growing in the new cultivation environment. That is why a tangible delay was not observed in the growth phase. C. raciborskii strains have striking compatibility with different conditions. According to the results, the growth of this cyanobacterium was probably under the influence of factors such as nitrogen saving capacity (due to heterocyst), while other algal groups faced nitrate scarcity (19, 20).
4.1. Conclusions
Cyanobacteria save phosphate in the growing phase that improves their growth. Thus, reduction and increase of phosphate content in the environment is important as a preventive factor of growth. Eventually, it should be noted that colonies of C. raciborskii are different in terms of physiological and genetic perspectives. Hence, it should be highlighted that results of other studies indicated that special colonies had considerable compatibility and other colonies showed different behaviors under such conditions. Overall, studying C. raciborskii and the effect of different phosphorus concentrations on the growth of these cyanobacteria were the reasons to conduct the current study. For future work, more influential factors such as temperature, light, nitrogen content, etc., on the dominant algae species will be investigated.