1. Background
Chronic periodontitis (CP) is an inflammatory disorder triggered by bacterial infection. Inflammatory conditions impair the surrounding tissues supporting the teeth and eventually result in tooth loss. Periodontitis is estimated to affect 10 - 30% of the world population. It is also associated with several systemic conditions, including cardiovascular diseases, type II diabetes, osteoporosis, and rheumatoid arthritis (1, 2). Among these comorbidities, the relationship between periodontitis and rheumatoid arthritis is an interesting topic that has been inadequately studied.
Rheumatoid arthritis (RA), an autoimmune disease, is characterized by symmetrical polyarthritis, which commonly involves the joints of small hands and feet. Untreated RA patients may develop structural damage and loss of function in their involved joints, which may cause considerable disability (3). Joint destruction occurs in 1% of the population and is accompanied by increased mortality and complications of systemic comorbidities. Lifespan may decrease if the heart and lungs are involved (4, 5). Rheumatoid arthritis treatment aims to control the pain caused by inflammation and to improve patients’ physical function and quality of life (6). Despite great medical progress, the main cause of RA has not yet been determined. Nevertheless, etiologies have been attributed to inflammatory processes, genetic and environmental factors, hormonal imbalance, elevated autoimmune particles at the inflammation site, and infections (2, 7).
Recent literature has revealed that periodontal disorders can be associated with rheumatologic diseases, particularly rheumatoid arthritis. Also, some risk factors are common between periodontitis and RA, especially smoking (3). Pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, lead to osseous destruction in both diseases. Porphyromonas gingivalis is the only known prokaryote expressing peptidyl arginine deaminase (PAD), and is the main pathogen related to periodontitis. This enzyme converts arginine into citrulline and induces citrullination (8). On the other hand, the specific immune response to citrullinated proteins is a key feature in RA pathophysiology. It is postulated that various citrullination mechanisms can disturb the immune system's tolerance, which predisposes susceptible individuals to develop an extended immune response to endogenous citrulline proteins (3). Citrullinated proteins could potentially cause an autoimmune response by forming anticitrullinated protein antibodies (ACPA), which are characteristic of RA (9, 10).
Although several studies have confirmed the association between periodontal disorders and RA (3, 10, 11), studies reporting the effects of RA severity on periodontal disease conditions are contradictory.
2. Objectives
This study aimed to investigate the relationship between the severity parameters of these two diseases.
3. Methods
In this study, 73 patients with RA were evaluated who were referred to the rheumatology clinic in 2021. This study was approved by the ethics committee of the Qazvin University of Medical Sciences, Qazvin, Iran (IR.QUMS.REC.1398.344). The diagnosis of RA was confirmed using the American College of Rheumatology (ARA) criteria. The inclusion criteria were the presence of Ramfjord teeth, absence of any other systemic diseases that presented similarly to periodontitis (i.e., diabetes mellitus), absence of a current pregnancy, and absence of a current or history of consuming any antibiotics in the last three months. Individuals smoking cigarettes were not included. Informed written consent was obtained from all patients prior to inclusion.
3.1. Data Collection
Data related to the severity of RA disease activity in patients were collected using the Disease Activity Score in 28 Joints (DAS-28) questionnaire (remission: ≤ 2/6 - Mild: 2/6 - 3/2 - Moderate: > 3/2 - 5/1 - Severe: > 5/1) (12). This scoring system measures the intensity or disease activity based on assessments of tenderness and/or swelling of 28 joints, ESR level, and the patient’s score in the Health Assessment Questionnaire (HAQ) (13). Periodontal disease status was assessed based on the measurement of Clinical Attachment Loss (CAL) and plaque index (PI) indices. CAL was measured in Ramfjord teeth via probing depth, and the Silness-Löe index was used for measuring PI (14, 15). In this regard, CAL is defined as the location of soft tissue with respect to the cemento-enamel junction (CEJ).
Mild periodontitis: CAL: 1 - 2 mm
Moderate periodontitis: CAL: 3 - 4 mm
Severe periodontitis: CAL: ≥ 5 mm (15)
PI: 0 No plaque
PI: 1 Plaque is made visible by using the probe after it has been moved across the tooth surface.
PI: 2 Moderate accumulation of the plaque
PI: 3 Abundance of the soft matter.
For measuring PI, the scores for four surfaces of the tooth were added and divided by four, then divided by six (Ramfjord teeth). The score was interpreted as below:
PI: 0 Excellent hygiene
PI: 0.1 - 0.9 Good hygiene
PI: 1.0 - 1.9 Fair hygiene
3.2. Statistical Analysis
All data were analyzed using SPSS software v.24. Descriptive data were presented by analyzing frequency, mean ± standard deviation (SD), and Median ± IQR range according to the nature of the variables. The normal distribution of variables was assessed using the Kolmogorov-Smirnov test. Qualitative variables were compared by the chi-square test. P < 0.05 was considered statistically significant.
4. Results
This study included 73 RA patients, with the majority (63 individuals; 86.3%) being female and 10 individuals (13.7%) being male. The study population age was 46.3 ± 11, with a range between 25 to 73 years. The median (IQR) length of disease duration and plaque index were [3 (1.25 - 8.5)] years and [2 (1 - 2)], respectively. In our study population, the severity of periodontitis disease was mainly mild (48 cases; 65.8%), while severe disease and non-periodontitis were only presented in 1.4% of the participants.
Regarding Rheumatoid Arthritis (RA), most of the patients scored moderate disease activity, with 47.9% of the patients, and the least common RA presentation class was mild disease activity, with 8.2% of the cases. The results of the state of oral hygiene (based on PI) showed that most of the patients had poor to fair oral hygiene, and only a few (2.7%) had excellent oral hygiene (Table 1).
| Variables | Frequency (%) |
|---|---|
| DAS28 | |
| Remission | 19 (26) |
| Mild disease activity | 6 (8.2) |
| Moderate disease activity | 35 (47.9) |
| High disease activity | 13 (17.8) |
| PI | |
| Poor hygiene | 39 (53.4) |
| Fair hygiene | 32 (43.8) |
| Excellent hygiene | 2 (2.7) |
Distribution of Disease Activity Score 28 and Plaque Index in patients (N = 73)
The majority of patients with mild and moderate periodontitis had moderate RA activity. In both genders, the prevalence of moderate disease activity was the most common. None of the male patients presented severe disease activity. In women, the least common RA severity was mild disease activity (Table 2).
| Variable | DAS28, No. (%) | P-Value | |||
|---|---|---|---|---|---|
| Remission | Low | Moderate | High | ||
| Gender | 0.394 | ||||
| Male | 4 (40) | 1 (10) | 5 (50) | 0 (0) | |
| Female | 15 (23.8) | 5 (7.9) | 30 (47.6) | 13 (20.6) | |
Comparison of DAS28 According to the Gender of Rheumatoid Arthritis Patients
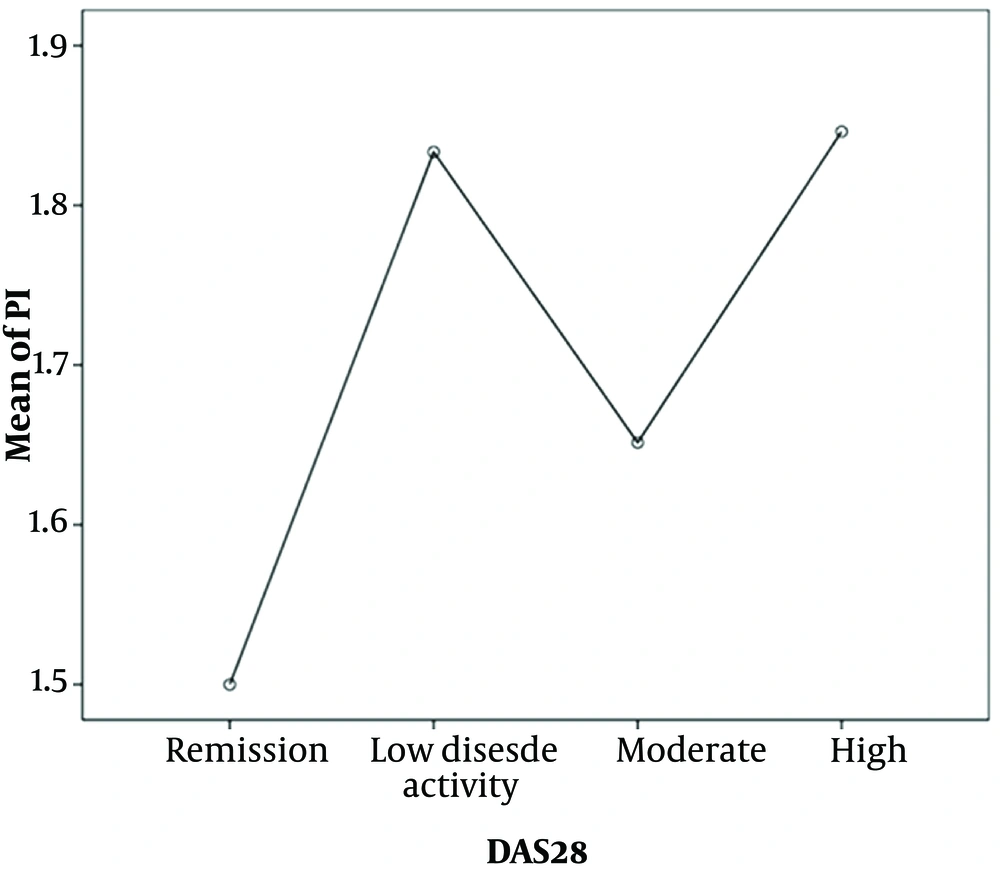
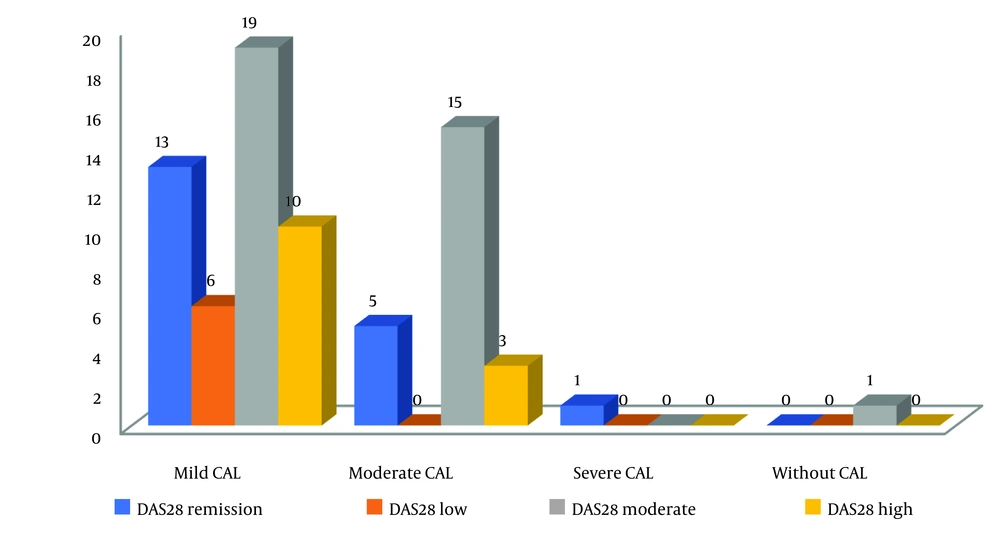
The patients with severe disease activity had the highest PI value, while the patients at the remission stage had the least PI level. However, this difference was not statistically significant (P = 0.574) (Figure 1). Although there was no relationship observed between the severity of DAS28 and CAL, in the mild and moderate categories of CAL, the frequency of moderate DAS28 was high in the studied patients (Figure 2).
5. Discussion
A total of 65.7% of our patients with RA had periodontal disease at the same time. Another study on periodontitis prevalence in RA patients reported a prevalence of 60% (15). The studies conducted by Bingham and Moni and Ayravainen et al. (17, 18) concluded that patients with RA have a lower level of periodontal hygiene and health than controls. This poor periodontal status could be due to weakened or suppressed host immunity as a result of RA and increased systemic inflammation of the whole body, which may predispose patients to the initiation or progression of periodontitis. In contrast, some studies have found no relationship between periodontal disease and RA (19-21). Sjostrom et al. (19) concluded that there were better periodontal conditions among RA patients, and severe periodontal disease occurred less frequently among RA patients (12%) than in the control group (16%). They found less plaque and calculus in RA patients, which shows a difference in periodontal care. These discrepancies may be explained by differences in the sample size, definition of periodontitis, age and sex factors, disease duration, type of drugs used, and other common factors contributing to the pathogenesis of these two diseases.
In the present study, 32.9% of the subjects had moderate to severe periodontitis, which did not correlate with higher RA disease activity (P = 0.372). In this study, 35 patients had moderate disease activity and 48 patients had mild periodontitis. Patients with a higher PI did not necessarily show higher RA disease activity. These findings are in agreement with the study conducted by Mobini et al. (15). They suggested no evidence of a link between periodontitis and the DAS28 scores. There was no association between RA disease activity and the number of teeth, CAL, PI, probing depth, or gingival index. In a study by Khantisopon et al. (20), a high prevalence of periodontitis in Thai patients with RA was found. However, there was no association between the RA parameters and periodontal conditions, which is consistent with the results of our study. Kordtabar et al. (21) found that there was no significant difference in rheumatologic indices between patients with and without periodontitis. They mentioned that they could not find a linear relationship between chronic periodontitis and the severity of RA.
However, Rodriguez-Lozano et al. (22) concluded that there is a significant association between RA disease activity and the severity of periodontitis. This may be due to the differences in the definitions of periodontitis severity degrees among the studies. This study defined the periodontitis grading as follows: Stage 0, intact healthy periodontium with interdental CAL (at the site of greatest loss) ≤ 3 mm; stage I, CAL ≥ 3 mm at the proximal sites of two or more non-adjacent teeth; and stage II, CAL ≥ 5 mm at ≥ 30% of teeth. In addition, they examined the whole oral cavity; but we examined only the Ramfjord teeth (22).
In this study, 47.9% of patients had moderate RA disease activity and 53.4% had poor PI. An increase in age is associated with a higher incidence of periodontitis. In this study, the patients had a mean age of 46.27 years. The ability of patients with RA to clean their teeth is lower than that of the normal population. This, in turn, may be the reason for the more likely plaque accumulation and increased gingival inflammation. Therefore, these patients require attention for oral hygiene control (18).
In the current study, most of the patients were female, which supports previous evidence of higher RA prevalence in women (23). There is a possibility of a relationship between RA and feminine hormones; lower levels of these hormones during menopause may increase the risk of disease. The protective effect of oral contraceptives on the RA risk remains controversial. On the other hand, there is established evidence showing that estrogen insufficiency affects periodontitis intensity. In menopausal women with osteoporosis, gingival recession, bleeding on probing, and CAL have been observed (24).
Smoking is one of the important risk factors for RA progression, and smokers have a deeper probing depth than those who have never smoked. Smoking affects the HLA-DR SE gene and increases the risk of anti-cyclic citrullinated peptide (anti-CCP) production in RA patients. Furthermore, smoking can result in plaque accumulation and thus negatively affect oral and dental hygiene (3). For this reason, smokers and those with systemic diseases such as diabetes were not included in this study.
The actual role of periodontitis in the pathogenesis of RA and its mechanisms are not fully understood. Nevertheless, the conditions and mechanisms of bone destruction in periodontitis and in RA are similar. P. gingivalis, the major pathogen related to chronic periodontitis, likely plays a key role in RA pathogenesis and protein citrullination (citrullination can disturb the immune system's tolerance and induce autoimmunity). P. gingivalis increases MMP-13, RANKL, IL-17, IL-1β, TNF-α, and CRP levels (24). In a study conducted by Correa et al. (25), the subgingival bacteria in RA patients and the control group were different. They reported that RA patients, even those with an intact periodontium, had a higher load of bacteria and pathogenic genus. Accordingly, probing depth and CAL were noticeably higher in RA patients. The microbial balance changes due to genetics, environment, and inflammatory factors. Chronic systemic inflammation, similar to RA, can negatively affect periodontal tissues, which in turn alters the microbial environment. A study showed that the levels of IL-2, IFN-γ, TNF, and IL-33 were significantly higher in RA patients without periodontitis than in the control group.
In addition, RA is an autoimmune disease with the characteristic auto-antibody of anti-CCP, which has diagnostic value and is synthesized by PAD. Considering the ability of P. gingivalis in PAD synthesis (PAD converts the protein arginine residue to citrulline), it is suggested that infection with this microorganism affects RA pathogenesis. Also, these citrullinated proteins have been detected in periodontal tissues, which indicates a strong association between these peptides synthesized in the oral cavity and those found in joints (24). However, in a study by Bialowas et al. (3), no significant difference was found between the RA and control groups in terms of P. gingivalis identified from specimens of periodontal pockets, but it had a relationship with periodontitis. They argued that P. gingivalis may increase the risk of RA or RA extent only in predisposed patients to periodontitis and other risk factors.
The periodontal condition could be influenced by RA-related medications. The low rate of severe periodontal lesions in patients with long-term disease may be explained by the beneficial effects of long-term treatment with anti-rheumatism drugs, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and biologic agents Drugs used to treat RA may have an impact on periodontal disease. The positive effects of long-term treatment with anti-rheumatism medications, such as nonsteroidal anti-inflammatory drugs and biologic agents, may be responsible for the low rate of severe periodontal lesions in patients with long-term illness. However, some studies have reported that biological agents enhance the risk of bacterial infections; in some cases, patients with advanced periodontal disease or progressive dental caries may need tooth extraction as prophylaxis for the prevention of odontogenic infection before starting biologic treatments. On the other hand, the use of corticosteroids in RA could potentially elevate infections due to immunosuppression. Steroid treatment results in decreased calcium absorption from the bowel tract, which can lead to bone demineralization. The majority of RA patients undergoing long-term treatment with corticosteroids are likely to have altered periodontal parameters (3). However, Ayravainen et al. (18), who studied the effects of different RA treatments on periodontal health, reported no significant changes in periodontal parameters compared to baseline levels.
Also, the patients suffering from RA had a lower number of teeth (26). It deserves to be mentioned that tooth loss is often a consequence of local inflammatory processes in periodontal tissues, and it is closely related to periodontitis. Some patients have lost all or some of their teeth. The currently available reports indicating a strong association between periodontitis and RA allow us to investigate whether the treatment of periodontal disorders could result in positive effects on RA disease activity. In a study conducted by Bialowas et al. (3), RA patients who had periodontal treatment showed reduced disease activity based on ESR and CRP levels. Periodontal disease is a local inflammation that not only leads to the destruction of the surrounding gingival tissues, which support teeth, but can be accompanied by a host systemic inflammatory response. Accordingly, it has been hypothesized that periodontal treatment can have beneficial effects on RA activity by reducing the levels of inflammatory markers. Bialowas et al. (3) reported no significant reduction in inflammatory markers, TNF-alpha, MMP-3, and MMP-9 levels after the periodontal treatment in RA patients. Nevertheless, improvements in VAS (Visual Analogue Scale) scores and the number of joints with tenderness and swelling were observed, which can decrease the DAS28 total score.
5.1. Conclusions
- The majority of our RA study population was composed of women.
- Most RA patients had mild periodontitis.
- The majority of patients with mild and moderate periodontitis had moderate RA activity. According to the prevalence of periodontitis in RA patients and its probable interference with the management of RA, periodic periodontal examination is suggested in these patients, and further studies with more participants are required to clarify the association.