1. Context
Poly cystic ovary syndrome (PCOS) is a complex endocrine disorder that affects women of reproductive age and is characterized by hormonal imbalance, ovulation disorders, and the presence of ovarian cysts (1, 2). It is estimated to affect approximately 4 - 8% of women worldwide and is one of the leading causes of infertility (3). Poly cystic ovary syndrome is a multifaceted disease with different clinical manifestations and underlying mechanisms, which are not yet fully understood (3). In recent years, extensive research has been conducted to explore the cellular mechanisms, metabolic factors, and associated risk factors of PCOS.
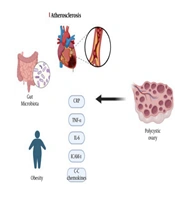
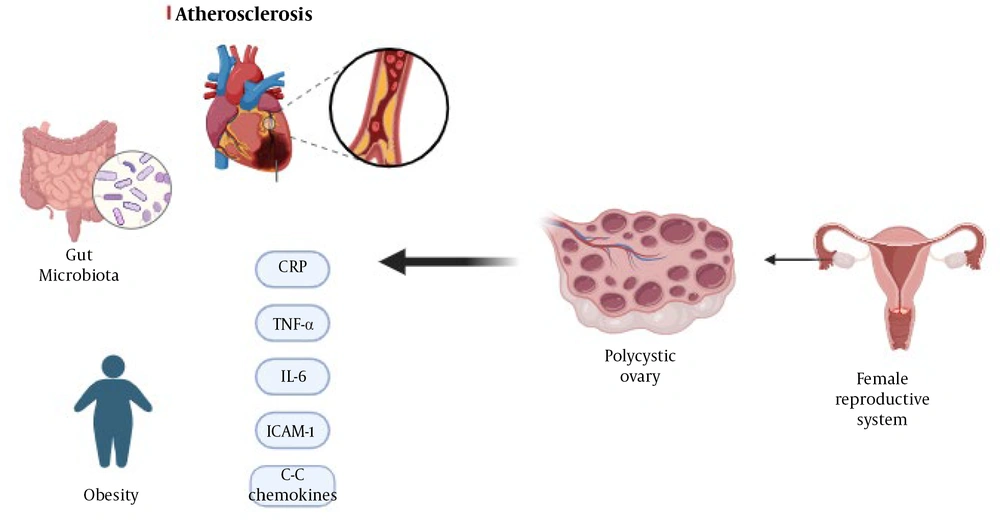
One prominent aspect of PCOS is the role of inflammatory mediators and pathways in its pathogenesis (1, 4). Emerging evidence suggests that dysregulated inflammation plays an important role in the development and progression of PCOS (4). In this context, several families of mediators have been investigated, including C-C chemokines such as CCL5 (chemokine ligand-5), which are significantly increased in women with PCOS compared to controls (4). In addition, interleukin-6 (IL-6) has been implicated in periodontal inflammation in PCOS, with elevated levels in the saliva of PCOS patients with periodontitis (5, 6). Furthermore, tumor necrosis factor alpha (TNF-α) has been identified as a key inflammatory mediator, with higher levels in PCOS patients, potentially contributing to metabolic disorders and insulin resistance. Hypothalamic inflammation, often driven by factors like obesity, plays a role in disrupting the delicate hormonal balance in the body, leading to irregular menstrual cycles and anovulation, two key features of PCOS (7). This inflammation can lead to insulin resistance, a hallmark of PCOS, which further exacerbates the condition by increasing blood glucose levels and triggering hyperinsulinemia (7). Insulin resistance can also lead to mitochondrial abnormalities, impacting ATP production and the electron transport chain (ETC), causing an energy imbalance within cells (7). Interestingly, disturbances in bile acid metabolism have recently been associated with PCOS, further highlighting the intricate web of factors contributing to the syndrome, ultimately affecting women's reproductive health and overall well-being (8). Recent research suggests that disturbances in bile acid metabolism may be linked to PCOS. Bile acids are not only involved in fat digestion and absorption but also play a role in regulating metabolic processes in the body (8). In individuals with PCOS, alterations in bile acid metabolism can potentially contribute to the disruption of metabolic homeostasis (8).
Cardiovascular events and risks are other important aspects of PCOS. Increasing evidence suggests that women with PCOS are at an increased risk for cardiovascular disease (CVD) compared to women without the syndrome (1, 9, 10). Carotid intima-media thickness (CIMT), serum sclerostin, and serum cardiotrophin levels can help determine cardiovascular risk (10-12). Some special conditions, like male equivalent PCOS and thyroid autoimmune diseases, which are relevant to PCOS mechanisms, can also contribute to cardiovascular risks (13, 14). Understanding the relationship between PCOS and cardiovascular health is essential because early identification and intervention can help reduce long-term risks.
This study is a narrative review article that was conducted by determining the relevant keywords based on MeSH and then searching the PubMed, Science Direct, ProQuest, and Google Scholar databases. Forty-one articles were used to write the present study. In light of these factors, this narrative review aims to provide an overview of the cellular mechanisms, metabolic factors, cardiovascular risks, biomarkers, and environmental influences associated with PCOS in the hope of leading to a better understanding of PCOS and providing insights for future research and clinical management strategies for this common and complex syndrome (Figure 1).
1.1. Inflammatory Mediators and Pathways in Poly Cystic Ovary Syndrome
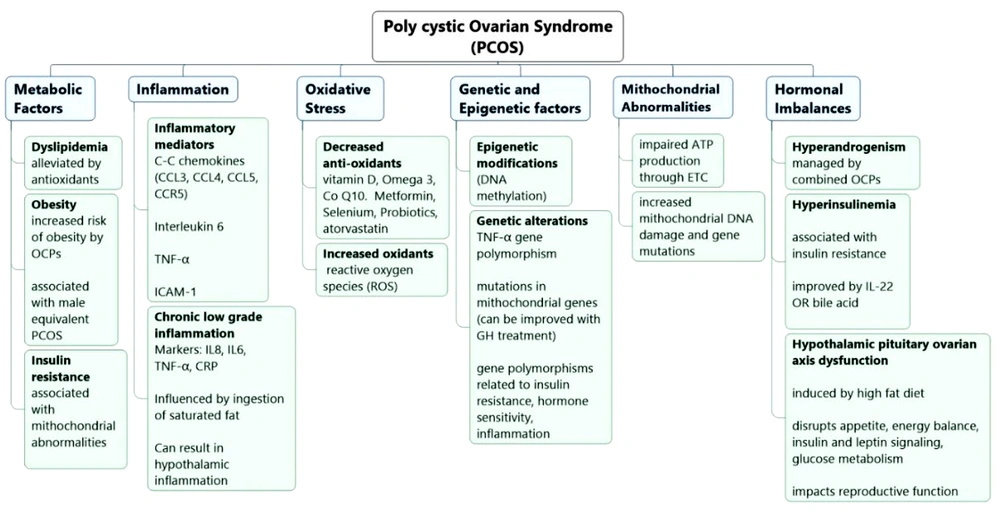
Poly cystic ovary syndrome is linked to both genetic and epigenetic alterations (15). Research has pinpointed genetic variations in genes related to insulin resistance, hormone sensitivity, and inflammation as potential contributors to PCOS development (15, 16). Additionally, epigenetic modifications such as changes in DNA methylation have been observed in PCOS, suggesting a potential epigenetic factor in PCOS susceptibility (4). This condition is also associated with chronic low-grade inflammation, which is implicated in its development and progression (17). Inflammatory markers such as C-reactive protein (CRP), IL-6, TNF-α, and IL-8 are elevated in PCOS patients, and this persistent inflammation may play a role in associated metabolic abnormalities (1). Certain chemokines, particularly CCL3, CCL4, and CCL5, and their receptor chemokine receptor-5 (CCR5) may be involved in the inflammatory processes and insulin resistance linked to PCOS (18). Studies have shown higher levels of CCL5 in women with PCOS compared to controls, along with increased CCR5 mRNA expression (18). However, further research is required to confirm these findings and elucidate the precise mechanisms at play. In a study exploring the relationship between IL-6 and PCOS, women with PCOS and periodontitis had notably higher levels of salivary IL-6, suggesting that periodontal disease may contribute to inflammation in PCOS patients (6).
Although TNF-α, a pro-inflammatory cytokine, is elevated in PCOS patients, its exact role and significance in PCOS remain uncertain. Genetic studies on TNF-α gene variations have produced conflicting results regarding their association with PCOS (4). Furthermore, saturated fat intake has been shown to increase inflammation, as indicated by the elevated levels of lipopolysaccharide (LPS), Toll-like receptor 4 (TLR-4), and TNF-α in obese women with PCOS (19). In contrast, lean women with PCOS exhibited increased TNF-α but suppressed LPS and TLR-4 responses after consuming saturated fat (19). These findings suggest that obesity plays a significant role in lipid-induced inflammation, meaning that obesity amplifies inflammation in PCOS, while PCOS itself worsens the inflammatory response.
1.2. Metabolic Factors and Risks in Poly Cystic Ovary Syndrome
The presence of increased oxidative stress and irregularities in the functioning of mitochondria have been noted in women with PCOS. Oxidative stress, which results from an imbalance between oxidants and antioxidants, contributes to the development of PCOS. Mitochondrial abnormalities, such as alterations in mitochondrial DNA copy number and mutations in mitochondrial genes, are linked to insulin resistance and other metabolic disorders in PCOS (9, 20, 21). Antioxidants have been the subject of research due to their role in reducing oxidative stress and alleviating the symptoms of PCOS (22, 23).
Substances like vitamin D, omega-3 fatty acids, coenzyme Q10 (CoQ10), metformin, selenium, probiotics, and atorvastatin have displayed promising effects in diminishing oxidative stress and enhancing various aspects of PCOS, including insulin sensitivity, lipid profiles, and ovulation (24). Vitamin D deficiency is common in women with PCOS and is linked to various aspects of this condition. Vitamin D plays a role in ovarian function, acts as an antioxidant, and influences insulin resistance, obesity, and reproductive outcomes. Vitamin D supplementation may have positive effects on liver function and fibrosis in overweight and obese women with PCOS who lack sufficient vitamin D. Nevertheless, further research is required to completely understand the effects and mechanisms of vitamin D in PCOS (24).
Growth hormone (GH) and IGF-1,2 (insulin-like growth factor) have been explored for their potential therapeutic benefits in PCOS. Studies have indicated that GH treatment can reduce oxidative stress, enhance mitochondrial function, and inhibit apoptosis in the ovarian granulosa cells of PCOS patients. However, additional research is necessary to fully grasp the underlying mechanisms and investigate the clinical implications of GH therapy in PCOS (21).
1.3. Reproductive and Hormonal Aspects of Poly Cystic Ovary Syndrome
Women with PCOS are at increased risk of experiencing negative pregnancy outcomes, including gestational hypertension, gestational diabetes, preterm birth, miscarriage, and perinatal death. These elevated risks are often linked to hormonal imbalances and metabolic factors that are associated with PCOS. Therefore, it is crucial for individuals with PCOS to closely monitor and effectively manage their pregnancies (25). To address PCOS symptoms, medications that regulate menstrual cycles, lower androgen levels, and alleviate issues like hirsutism and acne are commonly prescribed. However, it's important to consider potential metabolic issues and a higher susceptibility to conditions like obesity, type 2 diabetes (T2DM), cardiovascular diseases, and coagulation disorders when using hormonal contraceptives for PCOS (26).
Research suggests that smoking can negatively impact blood flow in the ovaries of women with PCOS, leading to reduced ovarian tissue perfusion. Therefore, smoking cessation is recommended to improve the fertility of women with PCOS (27). In men, there's a male equivalent of PCOS characterized by specific hormonal and metabolic patterns (13). Although it shares some similarities with PCOS in women, it manifests differently in men and may lead to symptoms like androgenism, hair loss, and metabolic irregularities, increasing the risk of cardiovascular diseases, metabolic syndrome, and atherosclerosis (13, 28, 29). Early diagnosis and lifestyle changes are essential for managing these risks. Table 1 presents a comparison between PCOS and its male equivalent with information gathered from the reviewed studies (13).
| Feature | Male Equivalent PCOS | PCOS |
|---|---|---|
| Metabolic pattern | Higher frequency of decreased insulin sensitivity; high risk of hyperglycemia, insulin resistance and DM II (diabetes mellitus type2); higher risk of metabolic syndrome; increased risk of carotid atherosclerotic plaques | More prevalence in reducing insulin sensitivity and metabolic abnormalities such as hyperinsulinemia and hypertriglyceridemia.; high risk of hyperglycemia, insulin resistance and DM II; more prevalence of metabolic syndrome and cardiovascular diseases; increased risk of atheromatous and carotid atherosclerotic plaques |
| Clinical signs and symptoms | Premature androgenetic alopecia (AGA); hypertrichosis; insulin resistance | Menstrual irregularity; hirsutism; acne |
| Hormonal profile | Testosterone levels below the normal range | Increasing levels of androgens such as testosterone |
| Other | More prevalence of hyperinsulinemia | High prevalence of hyperinsulinemia, hypertriglyceridemia, and hypertension, especially in siblings of women with PCOS |
Abbreviation: PCOS, poly cystic ovary syndrome.
Additionally, women with PCOS have a higher prevalence of autoimmune thyroid diseases (AITD) compared to the general population. The exact relationship between PCOS and AITD is not fully understood, but it is believed to involve immune system disorders, hormonal factors, genetic predisposition, and environmental factors. Even if there are no obvious symptoms related to thyroid dysfunction, it's advisable for women with PCOS to undergo screening for thyroid function and autoantibodies (14).
1.4. Cardiovascular Risks and Atherosclerosis in Poly Cystic Ovary Syndrome
Poly cystic ovary syndrome is considered a significant risk factor for CVD, irrespective of Body Mass Index (BMI) (30). The connection between PCOS and CVD is intricate and multifaceted, involving factors like hyperandrogenism, insulin resistance, obesity, dyslipidemia, and chronic low-grade inflammation (1, 31). Poly cystic ovary syndrome patients exhibit increased CIMT, which suggests the presence of subclinical atherosclerosis (32). This finding implies that PCOS may be linked to the early stages of arterial wall thickening and plaque formation. Among PCOS phenotypes, Types A and B, characterized by hyperandrogenism, exhibited the highest CIMT levels when adjusted for BMI, indicating a greater risk of atherosclerosis. Type A presents hyperandrogenism with symptoms such as hirsutism and irregular menstrual cycles, whereas type B includes hyperandrogenism and metabolic issues like insulin resistance. Type C is characterized by impaired ovulation. Phenotype classification aids in a better understanding of the diversity of PCOS and its associated cardiovascular risks (32).
Lean PCOS patients without metabolic syndrome display elevated serum levels of CT-1 (cardiotrophin-1), which are linked to testosterone, high-sensitivity C-reactive protein (hs-CRP), and CIMT levels. This suggests that CT-1 may contribute to heightened cardiovascular risk in PCOS, independent of insulin resistance and metabolic syndrome (12).
Additionally, higher serum levels of sclerostin, a protein associated with bone metabolism and vascular function, have been observed in PCOS patients (11, 33). However, no correlation was observed between sclerostin levels and CIMT (11, 33). Further research is needed to fully PCOS understand the relationship between sclerostin levels and cardiovascular risk in PCOS.
1.5. Biomarkers and Diagnostic Procedures in Poly Cystic Ovary Syndrome
The ratio of adiponectin to leptin serves as a valuable diagnostic indicator of PCOS. Research has demonstrated a significant distinction in this ratio between women with PCOS and those who are healthy. Utilizing this ratio exhibits greater sensitivity in predicting PCOS than using adiponectin or leptin alone (34, 35). Poly cystic ovary syndrome is linked to chronic low-grade inflammation, and inflammatory markers like CRP are elevated in individuals with PCOS. A study comparing women with PCOS to control subjects revealed higher CRP and lower albumin levels in PCOS patients, indicating a higher severity of chronic inflammation. The CRP-to-albumin ratio has a stronger association with PCOS than free androgens and insulin resistance, regardless of BMI (35, 36). These findings indicated that inflammation plays a significant role in the development and progression of PCOS. Further research is needed to explore the impact of inflammation on long-term health outcomes and assess interventions targeting inflammation in the management of PCOS.
Intercellular adhesion molecule-1 (ICAM-1) is an inflammatory and endothelial dysfunction marker. One study demonstrated elevated ICAM-1 levels in women with PCOS. Intercellular adhesion molecule-1 may function as a potential biomarker for PCOS diagnosis and identifying individuals at risk of T2DM development. Additionally, this study introduced insulin resistance as a predictor of ICAM-1 levels in PCOS patients, using statistical analysis and linear regression. Patients with both T2DM and PCOS exhibit higher levels of inflammatory markers. Serum ICAM-1 levels positively correlated with other inflammatory markers related to PCOS. Moreover, ICAM-1 levels, as determined through ROC analysis, displayed high accuracy in diagnosing PCOS and T2DM in PCOS patients with normal glucose tolerance (37).
1.6. Intestinal Microbiota and Insulin Resistance in Poly Cystic Ovary Syndrome
Gut microbiota, bile acids, and interleukin-22 (IL-22) play important roles in inducing insulin resistance and ovarian dysfunction in PCOS (38-40). Researchers conducted experiments using female mice and individuals with PCOS. They observed changes in the gut microbial composition in women with PCOS compared to controls. Transplantation of the gut microbiota from women with PCOS into mice results in impaired glucose metabolism, insulin resistance, ovarian dysfunction, and altered hormone levels. Transplantation of Bacteroides vulgatus produces metabolic and reproductive abnormalities similar to those observed in PCOS. Furthermore, treatment with bile acid or IL-22 improved insulin resistance, ovarian function, and hormone levels in a PCOS mouse model. Modulation of the gut microbiota, bile acids, and IL-22 could have therapeutic potential for treating PCOS. In addition, these studies introduced inflammation and oxidative stress as two factors causing PCOS and non-alcoholic fatty liver disease (NAFLD) (41) (Figure 2).
2. Conclusions
The findings of this study demonstrate the role of inflammatory mediators such as C-C chemokines, IL-6, and TNF-α in the pathogenesis of PCOS. Chronic low-grade inflammation appears to be a key factor in the development and progression of PCOS and is associated with adverse health outcomes. Cardiovascular risks are significantly increased in PCOS patients, regardless of their BMI. Subclinical atherosclerosis, characterized by increased CIMT, emphasizes the importance of early detection and intervention to reduce long-term cardiovascular risks. Elevated ICAM-1 levels indicate higher inflammation and endothelial dysfunction in PCOS patients, making it a promising biomarker for diagnosing PCOS and identifying individuals at risk of developing T2DM.
In addition, the gut microbiota is an important factor in the pathogenesis of PCOS. Changes in the microbial composition of the intestinal flora lead to insulin resistance and ovarian dysfunction. Modulation of the gut microbiota and administration of bile acids and (IL-22) have been introduced as potential therapeutic strategies for PCOS management.
Diagnosis of PCOS is challenging due to the lack of specific biomarkers, phenotypic heterogeneity, and variability in symptoms. A comprehensive evaluation considering clinical, hormonal, and metabolic factors is necessary, but the limitation in sample size and referral of patients to only one diagnostic center affects the generalizability of the findings. Considering the heterogeneity of the symptoms and underlying factors, personalized approaches are more preferable in the diagnosis and management of PCOS. This requires further research to investigate genetic and epigenetic factors, oxidative stress, hormonal contraceptives, and their impact on the pathophysiology of PCOS.
Overall, a deeper understanding of PCOS and its complexities is necessary to improve the diagnosis, management, and prevention of the associated risks. This review provides valuable insights into the multifaceted nature of PCOS and paves the way for future research and development of targeted interventions to improve the health and well-being of people with this common syndrome.