1. Background
Pregnancy is the most critical period of life for the fetus, during which the brain and other organs grow and differentiate. Therefore, harmful chemicals, biological agents, and toxins can affect the development and differentiation of the nervous system, leading to defects including behavioral and cognitive abnormalities, and can also have long-term effects on brain structure and function (1-3). Bisphenol-A (BPA), an endocrine-disrupting chemical, is widely present in the environment and has been used for decades as a key chemical in the industrial production of a wide range of devices and materials, including dental fillings, water and soft drink bottles, containers, the inner lining of canned foods, and PVC plastic (4, 5). Some studies have shown that BPA can leach into food under certain conditions (6). It has been reported that BPA was found in 96% of women examined at concentrations close to those that cause adverse effects in children (7). Bisphenol-A has adverse effects, such as changes in the function of some endocrine glands, including sex hormones and the thyroid (8). Reduced male fertility, prostate cancer, abnormalities in testicular sexual development, changes in the pituitary and thyroid glands, immunosuppression, neurological defects, and behavioral changes have been partly attributed to BPA exposure (9).
Previous studies have shown that perinatal exposure to low doses (50 μg/kg/day) of BPA affects the development of the central dopaminergic system, induces anxiety-like behavior, reduces the activity of the enzyme monoamine oxidase, and even affects the concentration of monoamines, particularly dopamine, in the brain (10). Parkinson's disease (PD) is a neurodegenerative disease that is the second most common age-related neurodegenerative disorder after Alzheimer's disease. The exact cause of PD is still unknown, but it is thought to be a multifactorial disorder resulting from the combined effects of genetic and environmental risk factors. The disorder occurs when certain regions of the brain lose the ability to produce dopamine (11). Based on numerous animal studies, exposure to BPA in utero and during the neonatal period (when most brain development occurs) can affect the development of the nervous system, particularly the dopaminergic organization, resulting in significant changes in the structure and function of the nervous system in adulthood (10, 12). Therefore, exposure to this pollutant may increase the sensitivity of dopamine neurons to pollutants and elevate the risk of developing PD in adulthood. However, few studies have been conducted in this area.
2. Objectives
The aim of this study was to evaluate the risk of experimental PD induced by 6-hydroxydopamine (6-OHDA) in rats exposed to BPA before and after birth.
3. Methods
3.1. Animals
Adult Sprague-Dawley rats (200 - 230 g) were purchased from the Razi Institute (Karaj, Iran). The animals were housed in a temperature-controlled room under a 12-hour light-dark cycle, with lights on from 7:00 a.m. to 7:00 p.m. All procedures involving animals were performed according to the guidelines of the Iranian Convention for the Protection of Vertebrate Animals used for experimental purposes, and the protocol was approved by the Ethics Committee of Qazvin University of Medical Sciences, Qazvin, Iran.
Two male rats were added to each cage and kept together for one week. Vaginal smears were checked every morning, and if pregnancy was confirmed, it was calculated as the first day of gestation. Pregnant mice were randomly divided into four groups: (1) control, with a normal pregnancy; (2) sham (solvent, DMSO); (3) prenatal BPA exposure (50 μg/kg); and (4) prenatal and postnatal exposure to BPA (50 µg/kg each). Bisphenol-A (Tocris Bioscience, Bristol, England) or its solvent was injected intraperitoneally into the mothers from days 8 to 18 of gestation and into the neonates from days 10 to 30 of life. Thirty days after birth, eight males were randomly selected from each group, placed in separate cages, and maintained under standard conditions of temperature, humidity, and food for up to 120 days.
3.2. Stereotaxic Surgery and Microinjection
The rats were initially anesthetized with ketamine and xylazine (100/10 mg/kg). A dose of 10 - 12 μg of 6-OHDA (Sigma, USA) was dissolved in saline containing 0.2% ascorbic acid and injected unilaterally into the right striatum according to the atlas of Paxinos and Watson. The coordinates used were AP: 0.2, L: -3.5, DV: -8, TB: -3.3. The injection was performed using a stereotaxic apparatus (Stoelting, USA) and a 10-μL Hamilton syringe. At the end of the injection, the needle was left in place for an additional 5 minutes and then withdrawn at a rate of 1 mm/min (13).
3.3. Apomorphine-Induced Rotational Test
Animals were initially given a 5-minute habituation period. This was followed by an injection of apomorphine hydrochloride (0.5 mg/kg, i.p., dissolved in saline, Sigma). One minute later, the number of full turns was counted at 10-minute intervals for 1 hour in a cylindrical container (28 cm in diameter and 38 cm in height). Contralateral and ipsilateral rotations (away from and towards the lesion side, respectively) were counted as positive and negative, and the net number of rotations was defined as positive minus negative. All tests were performed between 13:00 and 16:00 hours (14).
3.4. Elevated Body Swing Test
The elevated body swing test was performed prior to the apomorphine-induced rotation test. The animal was placed in a cylindrical container and habituated for 10 minutes to reach a neutral position, defined as having all four paws on the ground. The animal was then held approximately 2 cm from the base of its tail and lifted 2 cm above the resting surface. The animal was held on the vertical axis, defined as no deviation of more than 10° to either side. If the animal moved its head to either side of the vertical axis, a swing was recorded. Before attempting another swing, the animal was required to return to the vertical position for the next swing to be counted. Swings were counted over a period of 1 minute. Biased swinging behavior was calculated as follows: L / (L + R, %) for left-biased swings and R / (R + L, %) for right-biased swings (L = number of left-biased swings, R = number of right-biased swings) (15). Rotational and drug-free elevated body swinging behavior was assessed 1 week before surgery (baseline) and at 4 and 8 weeks after surgery. The swing test was performed at least one day before the rotation test. All tests were performed blind to the treatment group.
3.5. Cell Preparation
Animals were decapitated after being rendered unconscious by an injection of ketamine and xylazine (100/10 mg/kg, i.p.). Brain tissue was extracted, carefully washed with cold saline, and transported in special tubes containing 10% buffered formaldehyde for 24 hours. The dehydration phase was carried out using increasing concentrations of ethyl alcohol and transparency in xylene. The tissue was then kept in liquid paraffin at 60°C for 12 hours. Three-micron sections were cut using a microtome, stained, and examined by light microscopy (16).
3.6. Histological Study
For each animal, mesencephalic sections (intramural 2.9 - 4.2 mm) were examined using a previously described method (17). Nissl-stained neurons of the SNC were counted manually using a light microscope (400 × magnification). At least two sections representative of each of the four Paxinos-Watson planes (4.2, 3.8, 3.2, and 2.97 mm intramural) were examined by scanning the entire extent on each side. The counting was conducted blindly.
3.7. Statistical Analysis
Data were analyzed using SPSS version 21 software, with one-way ANOVA followed by Tukey's test. Data were expressed as mean ± SD, and a P-value of less than 0.05 was considered statistically significant.
4. Results
4.1. Apomorphine Induced Rotation Test
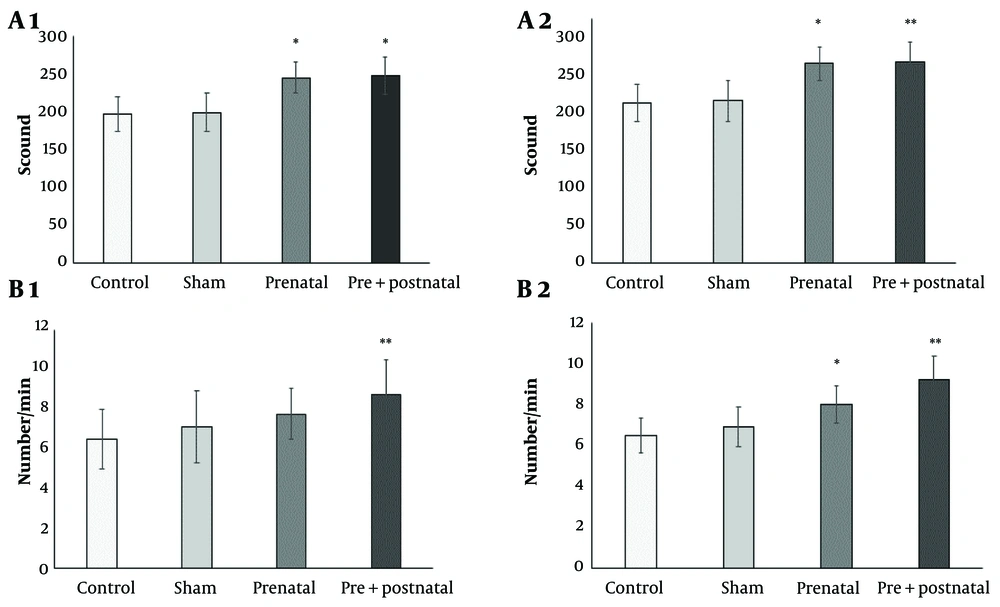
This test was performed twice (at the second and fourth weeks after the 6-OHDA injection). The number of spins induced by apomorphine in rats exposed to BPA during the prenatal (231.6 ± 25.6) and pre and postnatal (245 ± 26.4) periods was significantly higher than that in the control (180.1 ± 21.5) in both the second (Figure 1A1) and fourth weeks (245.6 ± 21, 247.2 ± 24.8, 197 ± 23.1, respectively) after the 6-OHDA injection (both P < 0.05) (Figure 1A2).
4.2. Elevated Body Swing Test
The results of the body swing test showed significant differences between the number of rotations per minute in the groups receiving BPA and the control group, particularly those receiving both pre- and postnatal BPA (P < 0.05, P < 0.01, respectively). The elevated body swing test results at the second week were as follows: Control 6.5 ± 1.5, prenatal 7.75 ± 1.26, and pre & postnatal period 8.75 ± 1.7 (Figure 1B1). At the fourth week, the results were: Control 6.9 ± 0.86, prenatal 8.05 ± 0.9, and pre & postnatal period 9.25 ± 1.15 (Figure 1B2) after the 6-OHDA injection.
4.3. Histopathological Evaluation of Nigral Part Compact Cell Density
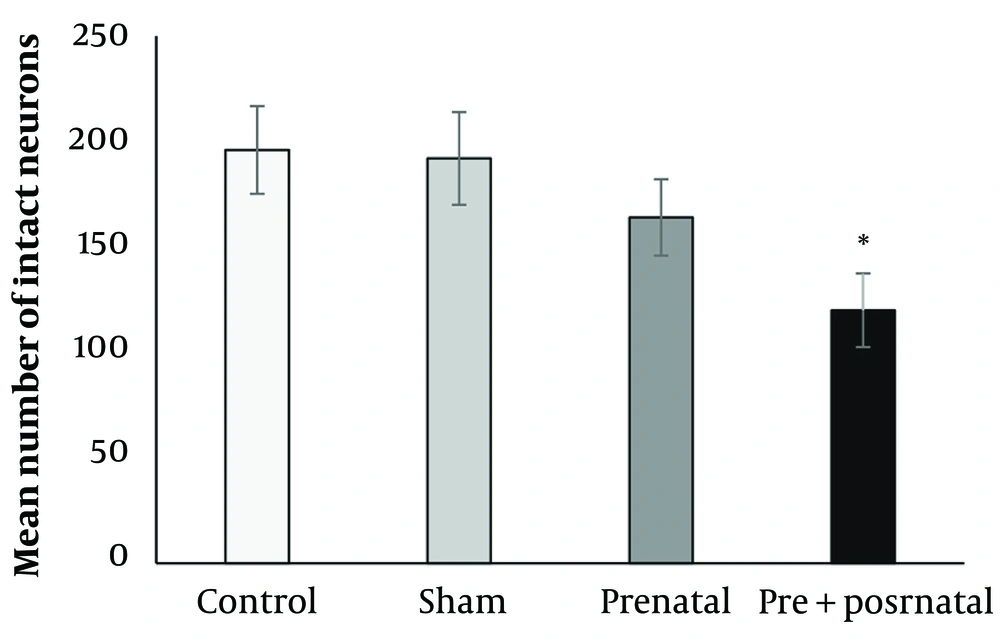
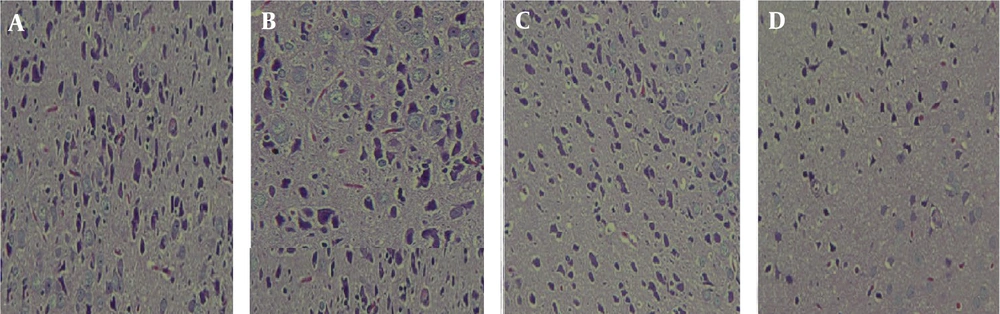
After staining the sections, the slides were examined under a light microscope 24 hours after drying. The results showed that BPA consumption significantly reduced the density of the compact part of the nigral cells (Figure 2). The mean number of neurons in rats exposed prenatally (164 ± 18.1) and pre- and postnatally (120.5 ± 17.5) to BPA was reduced compared to the control group (196.6 ± 20.8). The difference in the number of neurons in rats exposed to BPA pre- and postnatally was significant compared to the control group (P < 0.05) (Figures 2 and 3).
5. Discussion
This study investigated the association between pre- and/or postnatal exposure to BPA and the severity of 6-OHDA-induced PD in adult male rats. Behavioral and histological findings showed a significant increase in the severity of Parkinsonian behavior in the BPA-exposed group compared to the control group, particularly during the pre- and postnatal periods. The histological data also confirmed greater damage to nigral neurons in rats exposed to BPA during the embryonic and postnatal periods when exposed to 6-OHDA toxins. Our results are consistent with studies showing that BPA has a neurotoxic effect, particularly on dopaminergic neurons (17, 18). In this regard, several studies have confirmed the association between BPA exposure and post-pregnancy outcomes (19). In line with our work, a recent study has shown that exposure to BPA, even at the lowest human toxic concentration, can induce Parkinson-like disease in Drosophila (20). Additionally, transcriptional analysis of the brain of redfish exposed to BPA for 30 days showed that genes related to the dopaminergic pathway were significantly altered, leading to the disruption of dopaminergic processes and reduced dopamine levels (21). It has been reported that when BPA is added to cell culture, it can destroy noradrenergic, serotonergic, and dopaminergic neurons. As mentioned above, dopamine deficiency in the nigral region of the brain is the basic pathology of PD. Moreover, a reduction in noradrenergic neurons may make DA neurons more vulnerable (22). Exposure to BPA and its derivatives has been reported to reduce neurodevelopmental and psychomotor skills in children (23). Bisphenol-A exposure during lactation also affects growth parameters in 2-year-old children (24).
The results of this and previous studies show that exposure to BPA before and after birth accelerates and increases the susceptibility of dopaminergic nerve cells. Pregnancy and childhood are sensitive periods in which a species is more vulnerable to chemicals. Fetuses and children are less able to metabolize and excrete toxic substances than adults, so high levels of BPA in body compartments cause more damage (19). Based on recent studies, BPA is thought to cause oxidative stress by reducing antioxidant enzymes and increasing levels of malondialdehyde and reactive species (20). Bisphenol-A has also been reported to cause persistent adverse effects on neurodevelopment, such as neuronal differentiation and synapse formation, which persist into adulthood (25). Studies have shown that BPA exposure can affect thyroid and gonadal hormone signaling, which in turn affects normal brain development and subsequent behavior (23). Thyroid hormones play an important role in neuronal development through the myelination of axons and induction of synaptogenesis (26). On the other hand, levels of sex hormones are crucial in maintaining the proper functioning of brain circuits. It has been reported that a natural decline in estrogen levels in women or testosterone levels in men is associated with the onset of PD (27). Inflammation is an important part of the body's defense system, although excessive inflammation can also lead to various diseases. Neuroinflammation is implicated in the pathophysiology of PD (28). Bisphenol-A exposure exacerbated inflammation in a dose-dependent manner. Therefore, BPA may cause inflammation, leading to neurodegenerative disorders (29). The degree of involvement of these possible mechanisms in the results of the present study is unclear. Therefore, confirmation or rejection of all the mechanisms involved in Parkinson's susceptibility to BPA exposure certainly requires a more comprehensive study.