1. Background
The respiratory disease caused by SARS-CoV-2 is called Coronavirus disease 2019 (COVID-19) (1). The symptoms associated with COVID-19 vary from asymptomatic cases to a wide spectrum of symptoms such as fever, cough, tiredness, loss of taste or smell, and even death (2, 3). Epidemiological evidence suggests that the infection can spread through an infected patient’s respiratory secretions during speaking, coughing, or sneezing. Consequently, the World Health Organization (WHO) has recommended public use of masks, isolation, avoiding crowded spaces, social distancing, hand hygiene, room ventilation, and vaccination as necessary measures to control COVID-19 (3, 4). Effective vaccines for COVID-19 are crucial to reducing the enormous burden of SARS-CoV-2-related deaths (5). Given the ineffective treatments against COVID-19, vaccination has become one of the most important approaches to limit the epidemic (6). Vaccination is essential to prevent the COVID-19 pandemic and mitigate its adverse effects on public health (7).
The elderly, individuals with underlying conditions like cancer, cardiovascular disease, diabetes, and chronic respiratory disease, healthcare workers (HCWs), children, and pregnant women are more vulnerable to COVID-19 and are prioritized for receiving the vaccine. Among these groups, HCWs are particularly susceptible to the disease due to their high-risk exposure (3, 8-10). According to Chinese health centers, in 2020, 3,387 HCWs died of COVID-19 due to direct contact with infected patients in China and Wuhan (11). In this pandemic situation, access to personal protective equipment (PPE) for HCWs is crucial, but a shortage of PPE has disproportionately affected these groups (12). In Iran, HCWs, especially nurses, are the most exposed to coronavirus infection. Despite their higher knowledge, they have lower protection against the disease (13). For example, some did not use masks, gloves, or PPE, highlighting the necessity of improving protective measures for this susceptible group (14).
Globally, many types of vaccines have been produced, but evidence of their safety and potential side effects (SE) has not yet been fully confirmed (15). Therefore, it is critical to study vaccine complications during the general immunization stage. Additionally, because of the genetic diversity in different populations around the world, it is necessary to study the factors associated with COVID-19 vaccine complications in various populations. Certain signs and symptoms, such as injection site pain, headache, muscle pain, fatigue, fever, chills, and diarrhea, may occur after receiving the COVID-19 vaccination (16). Vaccine SE play a crucial role in the community's perception of the vaccination process.
Studies in Iran have shown that the most common SE were reported with the Sputnik, Oxford/AstraZeneca, and Covax vaccines. Muscle pain, fatigue, fever, and headache were the most common complaints among HCWs, and these SE were related to age and gender (5, 17, 18). The Sinopharm vaccine has relatively few adverse SE in Iran, while Sputnik's adverse effects, often after the second dose, included injection-site pain, hyperthermia, headache, asthenia, and joint pain (19). The SE of the AstraZeneca injection at the second dose were lower than those of the first dose, with the most prevalent complaints being headache, fever, muscle pain, fatigue, and joint pain (20). However, so far, few studies have been conducted on the factors associated with COVID-19 vaccine complications in Iran, making it necessary to conduct this study.
2. Objectives
This study aimed to evaluate the vaccination status and the associated factors in COVID-19 vaccine complications, including Sputnik V (Gamaleya Research Institute, Russia), Oxford/AstraZeneca vaccine (AstraZeneca, British-Swedish), Covaxin (Bharat Biotech, India), Sinopharm (National Pharmaceutical Group, China), Cov Iran Barekat (Barkat Pharmaceutical Group, Iran), and SpikoGen (CinnaGen and Vaxine, Iran-Australia) among HCWs in Chaharmahal and Bakhtiari province, Iran.
3. Methods
3.1. Population and Sample
This cross-sectional study was conducted from January to February 2022 in Chaharmahal and Bakhtiari province. Using the cluster sampling method, five cities, including Kuhrang, Saman, Borujen, Ardal, and Lordegan, were randomly selected out of a total of 10. The sample size was estimated based on the formula with a prevalence of 0.93 for vaccine complications (21), a 95% confidence interval (CI), and a mean estimation error (d) of 0.064, resulting in the inclusion of 739 HCWs. Individuals who had at least one year of occupational experience in a health care center and were willing to participate were selected.
3.2. Measuring Instruments
The information collected included age, sex, marital status, education and degree, work history, employment status, workplace status, underlying disease, history of coronavirus infection, history of hospitalization due to coronavirus, duration of hospitalization, availability of PPE, experience of COVID-19 infection, and history of vaccination against coronavirus and its complications. This data was gathered using a checklist.
3.3. Statistical Analyze
Continuous data are presented as mean ± standard deviation (SD), while categorical data are expressed as frequencies (%). To assess factors associated with vaccine complications, binary logistic regression was used to estimate the odds ratio (OR) and its 95% CI. Additionally, the OR was adjusted for age, gender, underlying disease, COVID-19 history, and vaccine type. A P-value of less than 0.05 was considered significant. All analyses were performed using SPSS software version 22.
4. Results
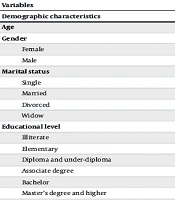
The mean ± SD age of HCWs was 38.78 ± 8.51 years. Additionally, the majority of HCWs (65.90%) were female. Approximately 43% of participants held a bachelor’s degree, and 19.90% had 10 to 15 years of work experience (Table 1).
| Variables | Values |
|---|---|
| Demographic Characteristics | |
| Age | 38.78 ± 8.51 |
| Gender | |
| Female | 487 (65.90) |
| Male | 252 (34.10) |
| Marital status | |
| Single | 176 (23.80) |
| Married | 550 (74.40) |
| Divorced | 9 (1.30) |
| Widow | 1 (0.50) |
| Educational level | |
| Illiterate | - |
| Elementary | 9 (2.1) |
| Diploma and under-diploma | 197 (26.7) |
| Associate degree | 138 (18.7) |
| Bachelor | 319 (43.2) |
| Master’s degree and higher | 76 (10.3) |
| Occupational Characteristics | |
| Employment status | |
| Informal | 378 (51.2) |
| Formal | 361 (48.80) |
| Work experience | |
| Under 5 years | 233 (31.50) |
| 5 to 9 years | 122 (16.50) |
| 10 to 15 years | 147 (19.90) |
| 15 to 24 years | 144 (19.50) |
| 25 years and above | 93 (12.60) |
| Workplace status | |
| Urban | 422 (57.10) |
| Rural | 317 (42.90) |
| Usually contact (most of the days of in a week) at work with suspected or confirmed COVID-19 patients. | 600 (81.20) |
| Appropriate personal protective equipment (PPE) to protect against Coronavirus disease 2019 (COVID-19) in the workplace | 520 (70.40) |
Demographic Characteristics of Participants (N = 739) a
Nearly 90% of HCWs had underlying diseases. Approximately half of the participants (370; 50.1%) had a history of COVID-19. The primary mode of COVID-19 transmission was reported to be the provision of services to an infected person at the workplace (Table 2).
| Variables | No. (%) |
|---|---|
| Transmission ways | |
| Providing service to an infected person at work | 172 (48.46) |
| Contacting an infected colleague at work | 48 (12.97) |
| Contacting an infected family member at home | 87 (23.52) |
| Public and crowded places | 18 (4.87) |
| Travel | 3 (0.81) |
| Unknown | 42 (11.35) |
| History of being hospitalized due to COVID-19 | 27 (3.7) |
| Hospitalization duration | |
| A week or less | 12 (44.44) |
| 1 - 2 weeks | 13 (48.14) |
| Two weeks to a month | 2 (7.41) |
Coronavirus Disease 2019 (COVID-19) Transmission Ways in the Participants and Their Relatives
Among vaccinated HCWs (712, 96.34%), nearly half (48.45%) did not report any complications after vaccination. The highest number of complications (176; 67.96%) was reported among those who received the Oxford/AstraZeneca vaccine, while the lowest number of complications (6; 35.29%) was reported among those vaccinated with the BIV1-CovIRAN vaccine. Information on complications after the COVID-19 vaccination is detailed in Table 3.
| Type of Vaccine | Values | No Complications | After the First Vaccination | After the Second Vaccination | After Both Vaccination |
|---|---|---|---|---|---|
| Oxford/AstraZeneca | 259 (36.37) | 83 (32.04) | 109 (42.09) | 19 (7.33) | 48 (18.54) |
| Sputnik V | 183 (25.70) | 89 (48.63) | 36 (19.67) | 12 (6.56) | 46 (25.14) |
| BBIBP-CorV | 218 (30.61) | 143 (65.60) | 31 (14.22) | 16 (7.34) | 28 (12.84) |
| BBV152 | 34 (4.77) | 18 (52.94) | 6 (17.65) | 3 (3.82) | 7 (50.59) |
| BIV1-CovIran | 17 (2.38) | 11 (64.71) | 4 (23.53) | - | 2 (11.76) |
| COVAX-19 | 1 (0.01) | 1 (100) | - | - | - |
| All vaccines | 712 (96.34) | 345 (48.45) | 186 (26.12) | 50 (7.02) | 131 (18.39) |
The History of Complications After the Coronavirus Disease 2019 (COVID-19) Vaccination by the Type of Vaccine a
After controlling for the effect of other variables, the odds of vaccine complications were 55% lower in men than in women (P = 0.001). Typically, frequent contact (most days of the week) at work with suspected or confirmed COVID-19 patients significantly increased the odds of COVID-19 vaccine complications (OR = 2.01, P = 0.021). A history of COVID-19 more than doubled the odds of vaccination complications (P = 0.001). The odds of experiencing vaccination complications were 2.32 times higher in HCWs who received Sputnik V compared to those who received Oxford/AstraZeneca (P = 0.002). In contrast, the odds of vaccination complications were 58% lower in individuals who received BBIBP-CorV compared to those who received AZD-1222 (P = 0.001) (Table 4).
| Variables | Odds Ratio (Confidence Interval) | |
|---|---|---|
| Crude | Adjusted | |
| Age | 0.99 (0.97 - 1.1) | 0.99 (0.97 - 1.01) |
| Gender (male) | 0.56 (0.41 - 0.77) c | 0.45 (0.32 - 0.64) c |
| Usually contact (most of the days in a week) at work with suspected or confirmed Coronavirus disease 2019 (COVID-19) patients. | 2.12 (1.42 - 3.17) c | 2.01 (1.30 - 3.11) c |
| Appropriate personal protective equipment (PPE) to protect against COVID-19 in the workplace | 0.90 (0.64 - 1.26) | 0.94 (0.65 - 1.35) |
| Underlying diseases | 1.01(0.64 - 1.58) | 0.97 (0.57 - 1.64) |
| Experienced COVID-19 infection | 1.62 (1.20 - 2.18) c | 2.03 (1.46 - 2.82) c |
| History of being hospitalized due to corona | 0.36 (0.15 - 0.84) c | 0.52 (0.21 - 1.30) |
| Hospitalization duration | ||
| A week or less | 1 | 1 |
| One to two weeks | 1.66 (0.29 - 9.44) | 1.12 (0.15 - 8.11) |
| Two weeks to a month | 2.66 (0.12 - 57.62) | 3.02 (0.12 - 72.27) |
| Type of vaccine | ||
| Oxford/AstraZeneca | 1 | 1 |
| Sputnik V | 2.00 (1.35 - 2.96) c | 2.32 (1.53 - 3.50) c |
| BBIBP-CorV | 0.49 (0.33 - 0.74) c | 0.42 (0.27 - 0.64) c |
| BBV152 | 0.51 (0.18 - 1.45) | 0.38 (0.13 - 1.10) |
| BIV1-CovIran | 0.84 (0.40 - 1.75) | 0.66 (0.31 - 1.42) |
5. Discussion
According to the results of this study, the highest vaccine complications were reported with Oxford/AstraZeneca, while the lowest were with BIVI-CovIran after the first dose. Additionally, vaccine SE were lower among men than women. Contact with an infected patient, a history of COVID-19 infection, and hospitalization for two weeks within a month were risk factors for COVID-19 vaccine complications. The majority of participants reported SE after the first dose, which aligns with previous studies. In a Turkish study, 62.5% of HCWs reported at least one SE after vaccination (22, 23).
The Oxford/AstraZeneca vaccine was associated with the highest prevalence of SE following the first dose (42.09%). In the studies by Babaee et al. and Babamahmoodi et al., Sputnik had more SE after the first dose than the second (15, 17). This inconsistency could be due to different sample sizes and study designs. However, Abu-Hamed et al. in Jordan conducted a study on vaccine SE among HCWs and found that the Oxford/AstraZeneca vaccine had more complications than Sinopharm and Pfizer at the first dose (24). In a cross-sectional study on the SE of the AstraZeneca COVID-19 vaccine, the prevalence of SE after the first dose was higher than after the second dose, consistent with our findings (20).
Among the three major vaccines in this study, BBIBP-CorV had the lowest complications after the first and both doses. A study in Jordan investigated Oxford-AstraZeneca, Pfizer-BioNTech, and Sinopharm SE among HCWs and showed that Sinopharm had the lowest SE, similar to our study (24). In the B.Q. study in the United Arab Emirates, participants experienced higher SE with the first dose of the Sinopharm vaccine than with the second dose; however, their participants were not HCWs (25). Another study showed that the adverse effects of the Oxford/AstraZeneca vaccine among HCWs were higher than those among non-HCWs after the first dose. This could be related to their workplace environment and educational background, as HCWs have clearly reported the SE of this vaccine (26).
Some studies have shown that SE are associated with young age, being female, and having comorbidities as independent factors for adverse events following immunization (5, 27). In our study, the relationship between SE, age, and underlying diseases was not significant, but SE was related to the female gender. Side effects is more common and intense in females due to a stronger immune response compared to males (28). Evidence confirms that women are at a higher risk of vaccine-related adverse events than men, which could be caused by the estrogen hormone triggering a robust immune response (29). Additionally, theories related to adaptive immunity, sex steroids, and innate immunity support this difference (30). The incidence of adverse events based on gender and contact history could be attributed to the type of population studied. Moreover, the type and immunogenicity of the vaccine may be linked to the symptoms of complications (31, 32).
In a cross-sectional study by Kadali et al., HCWs with a prior history of COVID-19 (PHC) had higher and more severe SE than those without PHC (33). Similarly, in the Mathioudakis study, the risk of any SE in patients with PHC infection was increased (34). These results align with our study. The number of HCWs with a PHC needing to take time off work increased, possibly related to vaccine SE. This may be connected to individual immune responses, with higher antibody titers after vaccination compared to those without a prior COVID-19 infection (35, 36).
In this study, COVID-19 vaccine SE increased if participants had previous exposure to infected patients. In Riad et al.'s study in the Czech Republic among HCWs, vaccine SE like injection site swelling, muscle pain, joint pain, and fever were more prevalent in HCWs exposed to infected cases (21). This may be due to the antibody response to COVID-19 in HCWs previously exposed to the virus through contact with infected patients (37).
5.1. Limitations
Healthcare workers were reluctant to participate in the study due to heavy workloads and the number of questions to answer. Filling out an online questionnaire may lead to information bias.
5.2. Conclusions
This study found that after receiving the COVID-19 vaccine, nearly half of the HCWs in Chaharmahal Bakhtiari experienced some complications. The Sputnik vaccine caused the most complications. Authorities should be alert and pay attention to the vaccines that have been imported.