1. Background
The burgeoning elderly population around the globe has resulted in increased susceptibility to developing neurodegenerative ailments (1). Of all existing neurological pathologies, Parkinson’s disease (PD) is considered the most rapidly progressing neurodegenerative disease, with increasing prevalence, disability, and death (2). Parkinson’s disease is characterized by the progressive loss of DA neurons in the substantia nigra pars compacta (SNpc) and a corresponding decrease in dopaminergic (DA) innervation in the striatum, leading to both motor and non-motor symptoms. The most common motor symptoms of PD include bradykinesia, resting tremor, postural instability, and limb rigidity (3, 4). Non-motor symptoms include hyposmia, depression, sleep disturbance, and autonomic dysfunction. Cardiovascular autonomic dysfunction is a common feature of PD, often characterized by blood pressure abnormalities that may occur early in the disease course and are more common in non-tremor-dominant patients. Another characteristic of PD is the buildup of ubiquitinated α-synuclein-containing aggregates within DA neurons. These accumulations, known as Lewy bodies, occur due to hindered axonal transport (5). At the onset of symptoms, approximately 30% of SN dopamine neurons and 50 - 70% of DA terminals in the striatum are lost, as observed in incidental Lewy body disease (ILBD). In postmortem brains of patients with this disease, numerous Lewy bodies are found despite the patient being asymptomatic while alive (6).
Currently, pharmacologic dopamine substitution is considered the most effective treatment for PD, but long-term administration of this medication can cause abnormal, uncontrollable, and involuntary movements (3). Rilmenidine, an imidazoline receptor agonist (7, 8), is an FDA-approved drug that has been identified as a mechanistic target of Rapamycin (serine/threonine kinase) (mTOR)-independent modulator of autophagy. This drug is widely used as an antihypertensive agent in humans and has no significant side effects. However, it is also known to stimulate autophagy by lowering intracellular cAMP levels (8), and improving motor symptoms in a mouse model of Huntington's disease (HD) (9).
2. Objectives
Considering the changes in blood pressure in PD and the fact that autophagy is one of the most important mechanisms in this disease, as well as the role of rilmenidine in the adjustment of blood pressure and autophagy, the present study investigated the effect of rilmenidine on DA neurons in the 6-hydroxydopamine model of PD in male Wistar rats.
3. Methods
3.1. Animals
Thirty male Wistar rats, weighing between 200 - 300 g, were obtained from the Razi Research Institute (Karaj). They were housed in groups of four per cage for one week to acclimate to the environment. During the course of the investigation, the rats were subjected to a balanced 12-hour light-dark cycle and were provided with ample access to food and water.
3.2. Rat Model of Parkinson Disease
The development of PD in a rat model was studied by randomly dividing the animals into three groups: Sham, lesion model, and treatment. Each rat was anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) combined with xylazine (20 mg/kg) and then carefully placed in a stereotaxic apparatus. Precise coordinates relative to the bregma were used as a reference point: Anterior-posterior (A/P): +0.2 mm, medio-lateral (M/L): 3 mm, and dorso-ventral (D/V): 4.5 mm from the dura at a flat skull position based on Paxinos and Watson's rat brain atlas. The left striatum region was targeted (10).
To create the PD model in rats, a solution consisting of 12.5 μg of 6-OHDA (Sigma, Germany) dissolved in 5 μL of ice-cold ascorbate saline (0.2%) was used within three hours after preparation. This solution was injected into the left striatum using a Hamilton syringe at a rate of 1 μL per minute. Following the injection, the needle remained inserted for five minutes to ensure proper distribution (11). In contrast, the sham group received injections containing only saline supplemented with ascorbate at equal concentrations into their respective left striatum regions.
The treatment group received 10 mg/kg rilmenidine (Sigma, USA) injected intraperitoneally four times per week (Mondays, Tuesdays, Thursdays, and Fridays) for four weeks following the lesion (9). To minimize mortality, postoperative care was provided during treatment (ethical code: IR.QUMS.AEC.1401.006).
3.3. Behavioral Test
The apomorphine-induced rotation test was conducted both prior to and four weeks following the procedure. Rats were administered an intraperitoneal injection of apomorphine hydrochloride (Sigma, Germany) dissolved in 0.9% saline at a dosage of 0.5 mg/kg. The rats were then observed within a transparent cylindrical chamber measuring 35 cm in diameter and 37 cm in height for a duration of 60 minutes, during which the total number of contralateral 360-degree rotations was documented (11).
3.4. Histological Examinations of Rat Brains
Upon completion of the behavioral tests, the rats were anesthetized and subjected to Nissl staining after transcardial perfusion with a solution of 4% paraformaldehyde and 0.5% glutaraldehyde diluted in phosphate-buffered saline (PBS; Gibco), which was maintained at a pH of 7.4. Subsequently, their brains were meticulously extracted from the skull and immersed in the same aforementioned solution for ten hours to undergo subsequent fixation procedures. Coronal tissue sections, each measuring ten micrometers thick, were taken specifically from within the SNpc region situated between interaural points spanning distances from approximately 2.4 mm to about 2.9 mm, as specified by the Paxinos atlas.
Coronal sections, spanning from the anterior to posterior regions of the SNpc pars compacta (SNpc), were delicately transferred onto slides coated with gelatin for each individual animal. These sections underwent staining with a 0.1% solution of cresyl violet, provided by Sigma in Germany. Neurons within the dense region of the SNpc were manually counted on sections aligned at four specific levels according to the Paxinos atlas measurements (2.96, 3.2, 3.8, and 4.2), while being compared relative to the central point of the interaural line under magnification of ×100. At least two sections were counted at each level, and neurons in the cytoplasmic area were also counted.
3.5. Statistical Analysis
All data are presented as mean ± standard deviation (SD). To analyze the results of Nissl staining of brain tissue and apomorphine-induced rotational behavior, a one-way ANOVA followed by a Tukey post hoc test was used. Additionally, a paired t-test was conducted to compare the results obtained both prior to and subsequent to the apomorphine-induced rotational behavior test and also to compare the number of cells in the right and left hemisphere. A statistical significance level was set at P < 0.05.
4. Results
4.1. Apomorphine-Induced Contralateral Rotations
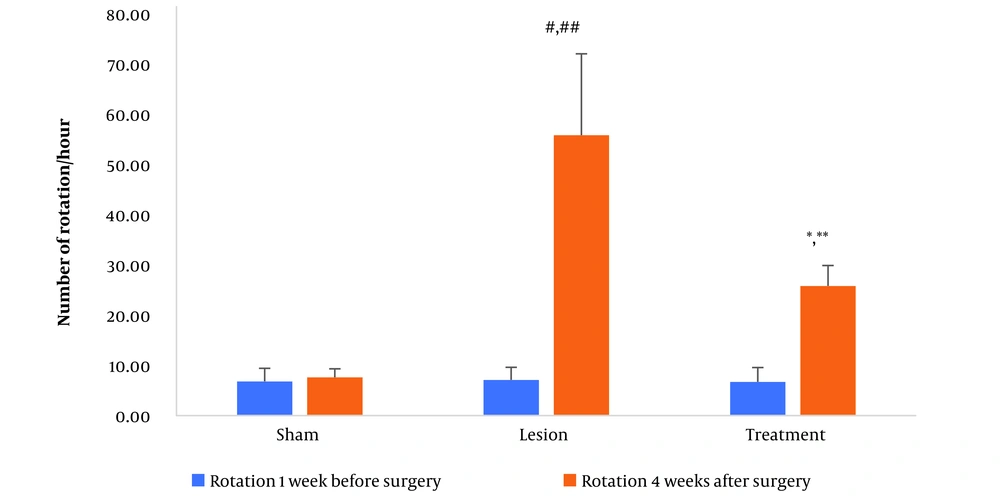
The apomorphine-induced contralateral rotation test was conducted one week before the surgical procedures (serving as a baseline) and four weeks after the surgery across the three groups of rats in this study. The mean number of contralateral rotations showed no significant difference among the groups one week before the procedure. However, four weeks post-surgery, the sham group displayed no discernible difference from their baseline state. In contrast, the lesion group exhibited a marked increase in the average number of contralateral rotations four weeks post-surgery compared to one week prior. Furthermore, there was a significant rise in the mean number of contralateral rotations within the lesion group compared to the sham group at four weeks post-surgery. Conversely, the treatment group demonstrated a notable decrease in the mean number of contralateral rotations four weeks post-surgery when compared to the lesion group (all P < 0.001, as depicted in Figure 1).
The data regarding apomorphine-induced rotations for three groups. Data are presented as mean ± SD. * vs. treatment group one week before surgery, ** vs. lesion group fourweeks after surgery, # vs. lesion group one week before surgery, and ## vs. sham group four weeks after surgery (all P < 0.001).
4.2. Nissl Staining of the Brain Tissues
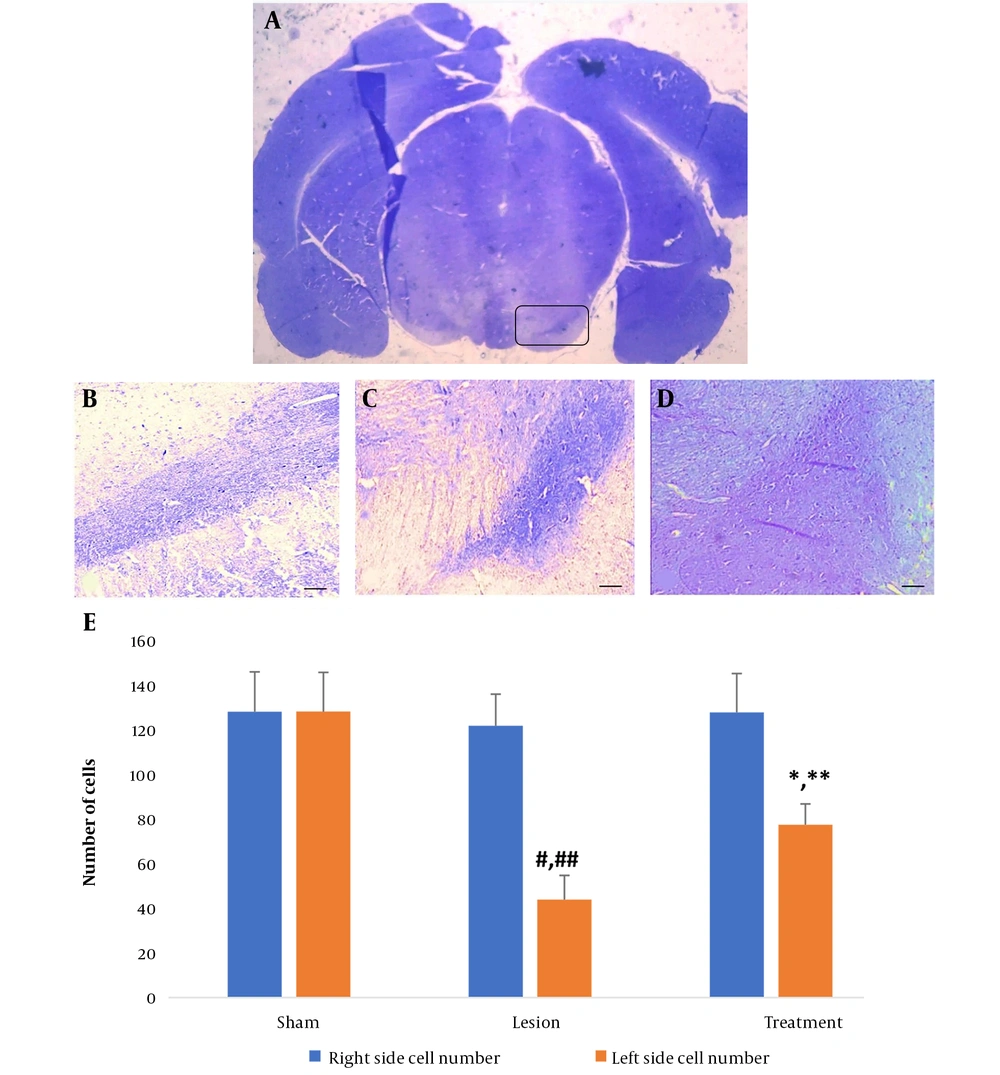
For visual examination purposes, microscopic images showcasing Nissl-positive cells in the SNpc of both hemispheres were provided for the three groups (as illustrated in Figure 2A-D ). No significant difference in the mean number of nigral cells in the right hemisphere was found among the three groups of rats. However, in the lesion group, there was a significant decrease in the average number of cells in the left hemisphere compared with the right hemisphere. Specifically, in the left hemisphere of the lesion group, there was a significant decrease in nigral cells four weeks after lesion induction with 6-OHDA compared to the sham group. In the treatment group, there was a significant increase in the number of nigral cells in the left hemisphere compared to the lesion group, although the number was still significantly lower compared to the right hemisphere (all P < 0.001, Figure 2E ).
Number of substantia nigra (SNpc) cells in all groups. A, coronal section of a whole-brain view under a stereomicroscope highlighting the SNpc (8X). The mentioned area is marked with a box. Light micrograph of the left SNpc in the B, sham; C, lesion; and D, treatment groups to count the dopaminergic (DA) neurons (100X magnification); E, number of SNpc cells in the left and right hemispheres in all groups. Data are presented as mean ± SD. * vs. treatment group right hemisphere, ** vs. lesion group left hemisphere, # vs. lesion group right hemisphere, and ## vs. sham group left hemisphere (all P < 0.001).
5. Discussion
The results of the present study showed a significant decrease in the number of rotations and a significant increase in SNpc neurons in the treatment group compared to the lesion group. Although no studies have specifically examined the effect of rilmenidine in PD, it has been used in other neurodegenerative diseases. Rilmenidine is a clinically approved antihypertensive agent that activates imidazoline-1 receptors (I1Rs) in the brain and periphery. Activation of I1Rs induces macroautophagy via a cAMP- and inositol trisphosphate (IP3)-dependent signaling pathway (12). Cardiovascular autonomic dysfunction, often characterized by blood pressure abnormalities, is a common non-motor symptom of PD (13). Thus, rilmenidine may be effective in PD, although further studies are needed.
Autophagy is a programmed cell death process necessary for removing long-lived organelles and damaged proteins. Impaired autophagy has been implicated in PD development, as it can hinder the clearance of α-synuclein, leading to its accumulation and misfolding. This misfolded α-synuclein may contribute to the formation of Lewy bodies, a characteristic feature of PD (14). The progression of PD is partly influenced by specific genetic mutations impacting intracellular transport pathways leading to the lysosome, suggesting autophagy's role in the disease's origin and development. Post-mortem examinations of brain tissues from individuals with sporadic PD have revealed an accumulation of autophagosomes and a simultaneous decrease in lysosomal markers, particularly within DA neurons (5). Autophagy is regulated by both MTOR-dependent and MTOR-independent pathways, and rilmenidine promotes MTOR-independent autophagic clearance. It has shown improvements in motor symptoms such as grip strength, tremor, and wire maneuvers in a mouse model of HD (9). Neurotoxicity induced by 3,4-methylenedioxymethamphetamine (MDMA) involves autophagy induction in 5 HT neurons in cell cultures. Rilmenidine potentially treats this disease's pathobiology through its beneficial effects on toxic intracellular events (15). However, the 2018 study by Perera et al. showed that despite robust macroautophagy (LC3-II levels) and efficient mitophagy induction, rilmenidine worsened the clinical course in the spinal cord of SOD1 mutant mice, a model of amyotrophic lateral sclerosis (ALS) (12). The number of autophagosomes is determined by the light chain of microtubule-associated protein 1 (LC3). LC3 is post-translationally processed to LC3-I and then converted to LC3-II, the only known protein that specifically associates with autophagosome membranes (9).
Rilmenidine appears to improve behavioral testing in the Parkinson's model by protecting SNpc neurons. According to research, the mechanism of action of this drug in other neurodegenerative diseases involves autophagy, which warrants further investigation in PD.