1. Background
Coronavirus disease 2019 (COVID-19) is an infectious disease that has rapidly spread worldwide (1). The disease is caused by the novel coronavirus SARS-CoV-2 (2). COVID-19 was first detected in Wuhan City, China, in December 2019 and was initially reported as a new type of pneumonia with an unknown cause (3).
In addition to causing a high death rate, the outbreak of COVID-19 significantly contributed to the collapse of the global economy, even before the implementation of quarantine programs worldwide (4). Health crises hampered economic activity during the outbreak and put governments in a difficult position (5).
While many countries continue to battle the COVID-19 crisis, some have successfully anticipated and implemented effective measures to slow the spread of the virus. Amid an international shortage of COVID-19 testing kits and medical equipment, South Korea (6) conducted extensive testing and set up screening clinics, helping to control the virus's spread early on. In contrast, Brazil and Russia, initially quiet at the start of the outbreak, are now experiencing crises in morbidity and mortality rates.
Fever and cough are the most frequent symptoms of COVID-19 (7), with fever reported in 88.7% of patients and cough in 67.8% (8). Other common symptoms include dyspnea, malaise, anorexia, anosmia, myalgia, and sometimes dizziness during hospitalization. Diarrhea may occur in 10% of cases (9). Less common symptoms (reported in less than 5% of cases) include heartburn, rhinorrhea, headache, chest pain, abdominal pain, and nausea (10).
Most people infected with COVID-19 (about 80%) experience a mild illness with mild clinical manifestations that do not require therapeutic interventions. About 15% of patients need medical intervention or hospital care, while another 5% develop acute illnesses that require intensive care (11).
For those requiring hospitalization, the average time from the first symptoms to the onset of dyspnea is 5 days (ranging from 1 - 10 days), the average time to hospitalization is 7 days (ranging from 4 - 8 days), and for those with more severe symptoms, the median time to acute respiratory distress syndrome is 8 days (ranging from 6 - 12 days) (7). Approximately one-quarter of hospitalized patients may need to be transferred to the intensive care unit (ICU) to manage complications such as hypoxemia or hypotension (12).
Current data suggest that elderly patients and those with comorbidities are at a higher risk of progression to severe disease and mortality. In a study from China involving more than 44,000 confirmed cases of COVID-19, the mortality rate for patients aged < 50 years was less than 0.5%. The most common clinical features in patients with severe disease include shortness of breath, severe dyspnea, lower initial oxygen saturation, and lymphopenia. In a predictive model, Chinese researchers identified four factors independently associated with disease progression during hospitalization: The presence of comorbidities, age > 60 years, lymphocyte count < 109, and elevated lactate dehydrogenase (LDH) levels (13).
2. Objectives
Given the ongoing pandemic and the importance of understanding the symptoms, underlying factors, and consequences of the disease, we aim to conduct further research in this area.
3. Methods
3.1. Study Design
This multicenter cross-sectional study was conducted to investigate the epidemiological characteristics, signs and symptoms, and outcomes of patients with COVID-19 who were hospitalized in 15 Iranian hospitals from February 4, 2020, to February 3, 2021. Data were extracted from the national registration system of the Medical Care Monitoring Center (MCMC). Permission to access the medical records of COVID-19 patients was provided by the relevant health officials.
The collected data included demographic information (age, gender, smoking status, place of residence, and citizenship), history of comorbidities (such as cancer, cardiovascular diseases, hypertension, diabetes, acquired immunodeficiency diseases, chronic kidney disease, pulmonary disease, blood disorders, chronic liver disease, and neurological disorders), clinical symptoms (fever, cough, myalgia, anosmia, ageusia, vertigo, headache, vomiting, nausea, diarrhea, cramps, anorexia, chest pain, limb paresis, and limb plegia), signs of the disease (loss of consciousness, respiratory distress, arterial oxygen saturation level, convulsions, skin lesions), duration of hospitalization, and the outcome of the disease (recovery or death).
3.2. Population
This study used convenience sampling to select participants. The study population included all patients diagnosed with COVID-19 who were admitted to 15 hospitals in the state of Qazvin, Iran, from February 4, 2020, to February 3, 2021.
3.3. Diagnosis
COVID-19 was confirmed through real-time PCR or by a physician using clinical evidence and radiological findings based on the Ministry of Health protocol.
3.4. Inclusion Criteria
Patients hospitalized with confirmed SARS-CoV-2 infection were included in this study.
3.5. Exclusion Criteria
Patients with incomplete medical records were excluded from the study.
3.6. Primary Outcome
The primary outcome of this cross-sectional study was death due to COVID-19 infection or complications related to the disease.
3.7. Statistical Analysis
The findings were presented using frequency tables, graphs, and numerical indicators. The Kolmogorov-Smirnov test was employed to evaluate the normality of the data. Chi-square and Fisher’s exact tests were used to analyze categorical data. Cox proportional hazards regression was utilized to analyze the survival data, with hazard ratios and 95% confidence intervals (CIs) estimated. Univariate analysis was conducted to identify factors affecting the outcomes of hospitalized patients, while multivariate analysis was performed to adjust for the influence (covariation) of other variables on the outcome variable. Data analysis was performed using SPSS Version 18 (SPSS Inc., Chicago, IL, USA) software, with a P-value of less than 0.05 considered significant.
3.8. Ethical Considerations
The study protocol was approved by the Institutional Ethical Committee of Qazvin University of Medical Sciences (IR.QUMS.REC.1399.009). All patient information was recorded confidentially and anonymously. The measures outlined in this study did not impose any additional costs on patient treatment. A checklist form was used to record the data.
4. Results
From February 4, 2020, to February 3, 2021, 18,653 patients were admitted to hospitals. A total of 838 patients (4.5%) were excluded from the dataset due to missing values, leaving data from 17,815 patients for analysis. Among these 17,815 hospitalized patients, 15,137 (84.9%) were discharged after recovery, while 2,678 (15.1%) died.
The median age (± IQR) of the patients was 62 (± 29) years, ranging from 7 to 103 years. The most commonly admitted age group was 60 - 79 years old. About 49.9% of the patients were female, and 50.1% were male. The death rate was slightly higher in men than in women (OR = 1.35, CI: 1.24 - 1.46, P < 0.001). Approximately 1.9% of hospitalized COVID-19 patients had a history of smoking. Most of the patients were residents of the city. There was no statistically significant relationship between the mortality rate of COVID-19 and a history of smoking, place of residence, or citizenship.
Cardiovascular disease, hypertension, and diabetes mellitus were the most common comorbidities among COVID-19 patients. The highest mortality rate was observed in patients with cardiovascular diseases. The mortality rate of COVID-19 was significantly associated with several comorbidities, including cancer (OR = 2.24, CI: 1.67 - 3.00), cardiovascular diseases (OR = 1.36, CI: 1.25 - 1.49), hypertension (OR = 1.37, CI: 1.24 - 1.51), kidney diseases (OR = 1.64, CI: 1.26 - 2.14), pulmonary diseases (OR = 1.48, CI: 1.22 - 1.79), and liver diseases (OR = 4.37, CI: 2.86 - 6.65) (P < 0.001) (Table 1).
| Variables | Total; (N = 17815) | Discharge; (n = 15137) | Death; (n = 2678) | P-Value |
|---|---|---|---|---|
| Age (y) | < 0.001 | |||
| < 20 | 414 (2.3) | 401 (2.6) | 13 (0.5) | |
| 20 - 39 | 2644 (14.8) | 2539 (16.8) | 105 (3.9) | |
| 40 - 59 | 4890 (27.4) | 4465 (29.5) | 425(15.9) | |
| 60 - 79 | 6752 (37.9) | 5518 (36.5) | 1234 (46.1) | |
| ≥ 80 | 3115 (17.5) | 2214 (14.6) | 901 (33.6) | |
| Gender | < 0.001 | |||
| Female | 8889 (49.9) | 7721 (51.0) | 1168 (43.6) | |
| Male | 8926 (50.1) | 7416 (49.0) | 1510 (56.4) | |
| History of smoking (yes) | 355 (1.9) | 291 (1.9) | 44 (1.6) | 0.326 |
| Place of residence | 0.375 | |||
| City | 12933 (72.6) | 10970 (84.8) | 1963 (15.2) | |
| Rural | 4882 (27.4) | 4167 (85.4) | 715 (14.6) | |
| Citizenship | 0.158 | |||
| Iranian | 17467 (98.1) | 14832 (84.9) | 2635 (15.1) | |
| Foreign | 348 (1.9) | 305 (87.6) | 43 (12.4) | |
| Underlying comorbidity | ||||
| Cancer | 224 (1.3) | 161 (1.1) | 63 (2.4) | < 0.001 |
| Cardiovascular disease | 4869 (27.3) | 3982 (26.3) | 877 (33.1) | < 0.001 |
| Hypertension | 3586 (20.1) | 2925 (19.3) | 661 (24.7) | < 0.001 |
| Diabetes mellitus | 2702 (15.2) | 2264 (15.0) | 438 (16.4) | 0.063 |
| Immunodeficiency disease | 39 (0.2) | (37) 0.2 | 2 (0.1) | 0.083 |
| Chronic kidney diseases | 323 (1.8) | 251 (1.7) | 72 (2.7) | < 0.001 |
| Chronic pulmonary disease | 665 (3.7) | 529 (3.5) | 136 (5.1) | < 0.001 |
| Chronic blood diseases | 98 (0.6) | 84 (0.6) | 14 (0.5) | 0.836 |
| Chronic liver diseases | 72 (0.4) | 51 (0.3) | 21 (0.9) | 0.001 |
| Chronic nervous disorder | 241 (1.4) | 202 (1.3) | 39 (1.5) | 0.615 |
| Other chronic diseases | 1034 (5.8) | 870 (5.7) | 164 (6.1) | 0.442 |
a Values are expressed as No. (%).
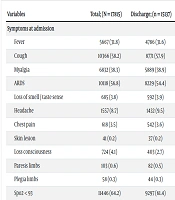
Fever, cough, myalgia, dyspnea, and Spo2 < 93 were among the most common signs and symptoms observed in patients. Approximately 80.2% of deceased patients had Spo2 < 93.
The mortality rate of COVID-19 was significantly associated with certain symptoms and signs at admission, including myalgia (OR = 0.854, CI: 0.78 - 0.93), acute respiratory distress syndrome (ARDS) (OR = 2.0, CI: 1.84 - 2.20), headache (OR = 0.469, CI: 0.39 - 0.57), loss of consciousness (OR = 4.98, CI: 4.27 - 5.80), Spo2 (OR = 2.55, CI: 2.31 - 2.82), and anorexia (OR = 0.60, CI: 0.52 - 0.70) (P < 0.001) (Table 2).
| Variables | Total; (N = 17815) | Discharge; (n = 15137) | Death; (n = 2678) | P-Value |
|---|---|---|---|---|
| Symptoms at admission | ||||
| Fever | 5667 (31.8) | 4786 (31.6) | 881 (32.9) | 0.19 |
| Cough | 10366 (58.2) | 8771 (57.9) | 1595 (59.6) | 0.118 |
| Myalgia | 6832 (38.3) | 5889 (38.9) | 943 (35.2) | < 0.001 |
| ARDS | 10118 (56.8) | 8229 (54.4) | 1889 (70.5) | < 0.001 |
| Loss of smell / taste sense | 685 (3.8) | 592 (3.9) | 93 (3.5) | 0.277 |
| Headache | 1557 (8.7) | 1432 (9.5) | 125 (4.7) | < 0.001 |
| Chest pain | 618 (3.5) | 542 (3.6) | 76 (2.8) | 0.053 |
| Skin lesion | 41 (0.2) | 37 (0.2) | 4 (0.1) | 0.344 |
| Loss consciousness | 724 (4.1) | 403 (2.7) | 321 (12.0) | < 0.001 |
| Paresis limbs | 103 (0.6) | 82 (0.5) | 21 (0.8) | 0.127 |
| Plegia limbs | 58 (0.3) | 44 (0.3) | 14 (0.5) | 0.052 |
| Spo2 < 93 | 11446 (64.2) | 9297 (61.4) | 2149 (80.2) | < 0.001 |
| Convulsion | 67 (0.4) | 61 (0.4) | 6 (0.2) | 0.163 |
| Cramp | 365 (2.0) | 318 (2.1) | 47 (1.8) | 0.244 |
| Nausea | 1162 (6.5) | 1004 (6.6) | 158 (5.9) | 0.157 |
| Vomiting | 808 (4.5) | 704 (4.7) | 104 (3.9) | 0.079 |
| Diarrhea | 366 (2.1) | 322 (2.1) | 44 (1.6) | 0.103 |
| Anorexia | 1902 (10.7) | 1711 (11.3) | 191 (7.1) | < 0.001 |
| Vertigo | 709 (4.0) | 615 (4.1) | 94 (3.5) | 0.177 |
| Inpatient ward and severity | ||||
| Ward | 15201 (85.3) | 13981 (92.4) | 1220 (45.6) | < 0.001 |
| ICU | 2614 (14.7) | 1156 (7.6) | 1458 (54.4) | < 0.001 |
| Ventilation | 1635 (9.2) | 371 (2.5) | 1264 (47.2) | < 0.001 |
| Duration of onset symptoms (day) | 0.188 | |||
| 1 - 5 | 11660 (65.5) | 9878 (65.3) | 1782 (66.5) | |
| 6 - 10 | 4885 (27.4) | 4169 (27.5) | 716 (26.7) | |
| 11 - 15 | 1031 (5.8) | 894 (5.9) | 137 (5.1) | |
| > 15 | 239 (1.4) | 196 (1.3) | 43 (1.6) |
a Values are expressed as No. (%).
The mortality rate was about 8.0% for patients in the isolated ward, 55.7% for those in the intensive care unit (ICU), and 77.3% for those who were mechanically ventilated (P < 0.001). The median duration from the onset of symptoms to hospital admission was 4 (± 3) days (Table 2).
Cox proportional hazard regression was used to identify factors influencing mortality among COVID-19 inpatients. The risk of death for COVID-19 inpatients increased with age, with patients over 80 years old having a 7.6 times higher risk of death than patients under 20 years of age. The risk of death from COVID-19 was also higher in men than in women.
Underlying comorbidities, including cancer, cardiovascular diseases, hypertension, chronic kidney disease, chronic pulmonary diseases, and chronic liver disease, were associated with an increased risk of death in hospitalized COVID-19 patients. Clinical symptoms such as acute respiratory distress syndrome (ARDS), headache, loss of consciousness, Spo2 < 93, and anorexia also increased the risk of death. However, the presence of myalgia reduced the risk of death by 10% compared to patients without this symptom (Table 3).
| Variables | Hazard Ratio (95% CI) | P-Value |
|---|---|---|
| Age group (Reference: Age < 20) | ||
| 20 - 39 | 1.73 (0.97 - 3.08) | 0.062 |
| 40 - 59 | 3.17 (1.83 - 5.51) | < 0.001 |
| 60 - 79 | 5.87 (3.39 - 10.14) | < 0.001 |
| ≥ 80 | 9.13 (5.28 - 15.78) | < 0.001 |
| Gender (Reference: Female) | ||
| Male | 1.21 (1.12 - 1.30) | < 0.001 |
| Underlying comorbidity | ||
| Cancer | 1.74 (1.35 - 2.23) | < 0.001 |
| Cardiovascular disease | 1.31 (1.21 - 1.42) | < 0.001 |
| Hypertension | 1.28 (1.17 - 1.40) | < 0.001 |
| Chronic kidney diseases | 1.57 (1.24 - 1.98) | < 0.001 |
| Chronic pulmonary disease | 1.60 (1.35 - 1.90) | < 0.001 |
| Chronic liver diseases | 2.33 (1.52 - 3.58) | < 0.001 |
| Symptoms at admission | ||
| Myalgia | 0.91 (0.84 - 0.98) | 0.019 |
| ARDS | 1.37 (1.26 - 1.49) | < 0.001 |
| Headache | 0.62 (0.52 - 0.74) | < 0.001 |
| Loss consciousness | 2.64 (2.35 - 2.97) | < 0.001 |
| Spo2 < 93 | 1.83 (1.66 - 2.01 | < 0.001 |
| Anorexia | 0.71 (0.62 - 0.83) | < 0.001 |
| Intubation | 4.86 (4.49 - 5.26) | < 0.001 |
The results of the multivariate analysis using the Cox proportional hazard regression model to determine the simultaneous effects of predictive factors on the risk of death in hospitalized COVID-19 patients are reported in Table 4. In this model, only factors with P-values less than 0.1 in the univariate analysis were included. According to the findings of the multivariate analysis, increasing age, chronic liver diseases, and intubation were associated with more than a twofold increase in the risk of death in hospitalized COVID-19 patients (P < 0.001).
| Variables | Hazard ratio (95% CI) | P-Value |
|---|---|---|
| Age group (Reference: Age < 20) | ||
| 20 - 39 | 1.833 (1.03 - 3.27) | 0.044 |
| 40 - 59 | 3.206 (1.84 - 5.58) | < 0.001 |
| 60 - 79 | 5.265 (3.04 - 9.11) | < 0.001 |
| ≥ 80 | 7.555 (4.36 - 13.09) | < 0.001 |
| Gender (reference: Female) | 1.115 (1.03 - 1.20) | 0.006 |
| Underling comorbidity (reference: No) | ||
| Cancer | 1.544 (1.20 - 1.99) | 0.001 |
| Chronic kidney diseases | 1.305 (1.03 - 1.65) | 0.027 |
| Chronic liver diseases | 2.109 (1.37 - 3.24) | 0.001 |
| Chronic pulmonary diseases | 1.399 (1.18 - 1.67) | < 0.001 |
| Symptoms at admission (reference: No) | ||
| ARDS | 1.193 (1.09 - 1.29) | < 0.001 |
| Loss consciousness | 1.486 (1.32 - 1.68) | < 0.001 |
| Headache | 0.809 (0.68 - 0.97) | 0.022 |
| Spo2 < 93 | 1.318 (1.19 - 1.45) | < 0.001 |
| Intubation | 4.004 (3.69 - 4.34) | < 0.001 |
5. Discussion
COVID-19 is a pandemic that has affected the entire world, leading to widespread outbreaks in nearly every country. This study investigated the epidemiological status of the disease across 15 hospitals from February 4, 2020, to February 3, 2021. During this period, approximately 18,653 cases of COVID-19 were admitted to hospitals. The results of the study indicated that age, underlying disease, and intubation were associated with an increased risk of death among COVID-19 patients.
The mean age of the patients was about 58.8 years, with a slightly higher number of infected males than females. A review by Peykari et al. found that the average age of COVID-19 patients in Iran was about 54 years, with 51.2% being male. This retrospective study included 113 confirmed COVID-19 cases admitted to hospitals in Shiraz City from February 20 to March 20, 2020 (12).
Our study showed that men were at a higher risk of death than women. The findings of another study also revealed that men die at a higher rate than women (14). However, the majority of research on COVID-19 patients has not found a significant gender difference in disease outcomes or mortality (15-17). Consistent with our findings, Salinas-Escudero et al. observed that male sex was an independent factor increasing the probability of COVID-19-related deaths (18). Men are more likely than women to be hospitalized for longer periods, which may be related to the fact that more men suffer from certain illnesses.
In the current study, cardiovascular disease, hypertension, and diabetes mellitus were the most common comorbidities, and the fatality rate was highest in patients with chronic liver disease. According to Nikpouraghdam et al., about 10.9% of patients had underlying diseases (13). The most common conditions were diabetes, chronic respiratory disease, hypertension, cardiovascular disease, chronic kidney disease, and malignancy. Most COVID-19 cases were seen in urban areas, which could be due to population density and frequent daily contact (19). However, 27.4% of the patients were from rural areas.
Our study found that patients with underlying comorbidities, such as cancer, cardiovascular diseases, hypertension, chronic kidney disease, chronic pulmonary (hypoxia) diseases, and chronic liver disease, had a higher risk of death compared to those without a history of these conditions. Further research indicates that people with COVID-19 who have underlying medical conditions, such as diabetes, high blood pressure, cancer, chronic respiratory diseases, or cardiovascular diseases, have an increased risk of severe illness and death (12, 20-22). Additionally, Bobdey S et al. investigated the survival and death rates of COVID-19 patients in a tertiary-care hospital in Maharashtra, India. They found that patients who required oxygen therapy and had multiple comorbidities had a much higher probability of death (17).
In the current study, the most common symptoms of the disease were fever, cough, myalgia, and ARDS, which are consistent with findings from other studies (23, 24). The fatality rate among hospitalized patients was about 15%, whereas in the Shahriarirad et al. study, the fatality rate was 8% (9 out of 113 cases), with most deaths occurring among ICU-admitted patients (14). In the Nikpouraghdam et al. study, 239 deaths occurred, resulting in a fatality rate of 1.85%, based on the total number of patients (both outpatient and inpatient) (13). About 14.7% of patients in the current study were admitted to the ICU, and their condition was severe. The mortality rate of ICU patients was about 64%.
Patients with mild illness (about 80%) can often be managed in the community if they are able to quarantine. They need to monitor their condition, recognize severe symptoms that would require hospital care, and manage any concerns they have. Care strategies should be tailored to the patient's condition. Patients whose home environments are unsuitable for safe management or infection prevention may require hospitalization. Discussions with public health officials are essential to ensure that quarantine mechanisms are in place and that appropriate follow-up is conducted (12). During times of high demand for healthcare, such as during a pandemic, safely managing low-risk patients in the community is necessary to maintain hospital capacity for severely ill patients.
Moderate to severe COVID-19 patients often need hospitalization. Severity factors include dyspnea with exercise, tachypnea at rest (respiratory rate > 22 breaths/minute), hypoxia (oxygen saturation < 93%), hypotension (systolic blood pressure < 100 mmHg), loss of consciousness, and acute respiratory distress syndrome, as observed on imaging. Patients with severe illness, characterized by a respiratory rate > 30 breaths per minute, oxygen saturation < 93%, and persistent hypotension, should be hospitalized and provided with intensive care (18, 25).
In our study, increasing age, chronic liver diseases, and intubation were associated with a more than twofold increase in the risk of death among hospitalized COVID-19 patients. In a hospital-based study in Ethiopia, Kaso et al. performed a survival analysis of COVID-19 patients. Their findings, which align with our own, indicated that being older was an independent risk factor that more than doubled the chance of dying from COVID-19. Elderly persons typically require extra care, especially if they have more severe conditions or underlying disorders (22).
To control the disease, it is important to follow personal hygiene recommendations, maintain social distancing, use masks, wash hands regularly, avoid staying in closed spaces for extended periods, refrain from attending gatherings, and get vaccinated. Evidence shows that vaccination is an effective method for preventing the acute form of COVID-19 and saving millions of lives by controlling the disease and reducing mortality (26).
Careful planning is essential for hospital administrators and policymakers to manage hospital beds effectively and reduce the length of stay (LOS) for COVID-19 patients. To decrease mortality rates and LOS, it is important to obtain a comprehensive medical history of COVID-19 patients and provide early, appropriate, and sufficient medical care. Since comorbidities and underlying illnesses can extend the duration of stay and increase mortality risk, ensuring that hospital staff are knowledgeable about these conditions may be beneficial during treatment. Based on the experience from the COVID-19 pandemic, focusing on mitigation efforts for individuals at higher risk of fatality could also be advantageous (27).
5.1. Conclusions
Based on the present study, advanced age, underlying diseases, and intubation increase the risk of death in patients with COVID-19. More than two years into the COVID-19 pandemic, the world continues to face various health, economic, social, and political challenges. Epidemiological studies are essential for the prevention and control of the disease. Ensuring optimal infection prevention is crucial from the moment a patient is diagnosed with suspected COVID-19 symptoms. This approach can help mitigate specific challenges for healthcare workers using personal protective equipment and for patients who need to manage quarantine issues.