1. Background
The liver is essential for energy exchange and the biotransformation of xenobiotics, as it is the first organ to come into contact with most digestion products. Damage to the liver disrupts normal metabolism and can even result in liver failure (1, 2). Drug-induced toxicity is a frequent cause of liver injury, accounting for approximately half of the cases of acute liver failure, and it can mimic all types of both acute and chronic liver disease (3, 4). It is also one of the most common reasons for medication withdrawal (3) and the most common cause of ending drug development programs (5).
Carbon tetrachloride (CCl4) is a typical hepatotoxic chemical commonly used to study the pathological mechanisms of liver injury and assess the hepatoprotective effects of novel drugs (6). Carbon tetrachloride reduces the activity of antioxidant enzymes and generates free radicals, leading to hepatocyte injury, both in vitro and in vivo (7). The metabolism of CCl4 into trichloromethyl (CCl3) and peroxy trichloromethyl (OOCCl3) free radicals is responsible for its toxicity. This process occurs in the endoplasmic reticulum and is mediated by the cytochrome P-450 oxidase system (8). Subsequently, OOCCl3 covalently binds to cellular macromolecules in the cell membrane, resulting in functional and morphological modifications that cause fat degeneration, fibrosis, cirrhosis, cell death, cancer, and hepatocyte necrosis (9).
Treating diseases that affect the liver is important and requires caution. Few traditional treatments are available without hepatotoxic side effects, and modern medicine has little to offer for liver disease treatment (10). It is vital to investigate safe and hepatoprotective drugs, taking traditional medical knowledge into account (11). Fructus Psoraleae, one of the most well-known traditional Chinese medicines, is made from the dried ripe seeds of Psoralea corylifolia L. (Leguminosae) and has been used extensively to treat a variety of illnesses, such as vitiligo, bone fractures, and osteoporosis (12). Bavachinin (BVC), isolated from P. corylifolia (13), exerts a variety of pharmacological actions, including antitumor, anti-inflammatory, antioxidative, antibacterial, antiviral, immunomodulatory, and anti-Alzheimer’s disease effects (14-18). Additionally, it has been demonstrated to be a natural pan-PPAR agonist that lowers blood levels of triacylglycerol and glucose (18, 19).
Based on increasing reports on the use of traditional medicine in the treatment of diseases (10, 20), it is imperative to determine the potential of BVC in the treatment or prevention of drug-induced toxicity.
2. Objectives
Moreover, due to the antioxidant and anti-inflammatory effects of BVC, this study aimed to investigate the potential effects of BVC in decreasing damage and oxidative stress in the livers of rats treated with CCl4.
3. Methods
3.1. Chemicals
Carbon tetrachloride and BVC (SMB00100) were purchased from Sigma-Aldrich Company (Sigma Aldrich Co., USA). Olive oil was used as the vehicle for BVC in intraperitoneal injections. Potassium citrate buffer (1%) was prepared by dissolving potassium citrate in water to achieve a final concentration of 1%. This buffer was chosen as the solvent for BVC for intraperitoneal injections because it can maintain a stable pH environment, which is crucial for the stability and bioavailability of BVC. Additionally, potassium citrate buffer is compatible with biological systems and is non-toxic, making it suitable for in vivo use without causing adverse effects on the experimental subjects (21).
3.2. Animals
Twenty-eight adult male Wistar rats, weighing 200 - 250 g, were provided by the Razi Vaccine and Serum Research Institute (Karaj, Iran). The rats were randomly assigned to four experimental groups. Randomization was done by generating random numbers in Excel, sorting the rats by these numbers, and then assigning them to groups sequentially. The animals were kept in separate cages under a 12-hour light/dark cycle at a temperature of 22 ± 2°C, with free access to food and water. The experimental protocol was approved by the Research Ethics Committee at Qazvin University of Medical Sciences with the ethics code IR.QUMS.REC.1399.552. This study was conducted in accordance with international, national, and institutional protocols for animal experiments.
3.3. Experimental Groups
The animals were randomly divided into four groups, each consisting of seven rats, and were treated for four weeks as follows:
(1) Control group: Potassium citrate buffer was administered via intraperitoneal injection for 4 weeks. Olive oil, serving as the BVC carrier, was also administered by intraperitoneal injection twice a week for 4 weeks.
(2) Bavachinin group: BVC was administered at a dose of 5 mg/kg through intraperitoneal injection once daily for 4 weeks (19).
(3) Carbon tetrachloride group: Carbon tetrachloride, mixed with olive oil in a 1:1 ratio, was administered at a dose of 1 mg/kg via intraperitoneal injection twice a week for 4 weeks.
(4) Treatment group: BVC at a dose of 5 mg/kg was administered once daily, and carbon tetrachloride mixed with olive oil (in a 1:1 ratio) at a dose of 1 mg/kg was administered twice a week via intraperitoneal injection for 4 weeks. The infusion of BVC began immediately after the first dose of carbon tetrachloride, with a 2-hour interval between the injections of BVC and CCl4. This interval allows BVC to effectively mitigate the liver damage caused by CCl4, ensuring that BVC reaches an optimal concentration in the liver to activate its protective mechanisms, such as antioxidant and anti-inflammatory effects. This timing strategy is supported by studies indicating that the timely administration of protective agents significantly reduces liver damage (22).
3.4. Analysis of Enzymes Indicative of Hepatic Functions
Blood samples were collected on the 14th and 28th days following BVC and CCl4 administration and were kept at room temperature for 15 minutes to allow for coagulation. The samples were then centrifuged at 4000 rpm to separate the serum, which was stored in a refrigerator for subsequent biochemical tests. The activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) were measured using specific assay kits from Pars Azmun Company (Karaj, Alborz, Iran) (23). The ALT Assay Kit (Code: 2 3009) and AST Assay Kit (Code: 2 3019) employ the IFCC method with pyridoxal phosphate activation, while the ALP Assay Kit (Code: 2 3034) uses a kinetic method.
3.5. Measuring the Amount of Malondialdehyde (MDA) in Liver Tissue
Malondialdehyde, a byproduct of lipid peroxidation, was measured in liver tissue samples using the thiobarbituric acid reactive substances (TBARS) method. To perform this, liver tissue samples were first homogenized by weighing a precise amount (e.g., 100 mg) and placing it in a chilled homogenization buffer, typically phosphate-buffered saline (PBS) with a pH of 7.4, at a ratio of 1 mL of buffer per 100 mg of tissue. The tissue was homogenized on ice using a mechanical homogenizer until a uniform consistency was achieved. The homogenate was then centrifuged at 10,000 g for 10 minutes at 4°C to remove cellular debris, and the supernatant was collected for the TBARS assay.
To prepare the necessary solutions, 30 mL of concentrated phosphoric acid (85%) was added to 970 mL of distilled water in a 1-liter volumetric flask, mixed thoroughly, and topped up to 1 liter to make a 3% solution. For the thiobarbituric acid solution, 0.6 grams of thiobarbituric acid was dissolved in 100 mL of distilled water, with gentle heating and stirring to ensure complete dissolution. These steps ensured the correct concentrations for the TBARS assay.
In the TBARS assay, 3 mL of 3% phosphoric acid and 1 mL of 0.6% thiobarbituric acid were added to 0.5 mL of the homogenate in a centrifuge tube. The mixture was heated for 45 minutes in a boiling water bath. After cooling, 4 mL of n-butanol was added, and the mixture was centrifuged for 20 minutes at 20,000 g. The absorbance of the organic phase (n-butanol) was then measured at a wavelength of 535 nm. 1,1,3,3-Tetramethoxypropane was used as the standard. The results were expressed as nanomoles of MDA per gram of liver tissue (nmol/g) (24).
3.6. Statistical Analyses
Significant differences were determined using a one-way analysis of variance (ANOVA) followed by the Tukey test, conducted with GraphPad Prism software, version 9 for Windows (GraphPad Software, San Diego, CA, USA). The results are presented as mean ± SEM for each rat group (n = 7). P-values of < 0.001, < 0.01, and < 0.05 were considered statistically significant.
4. Results
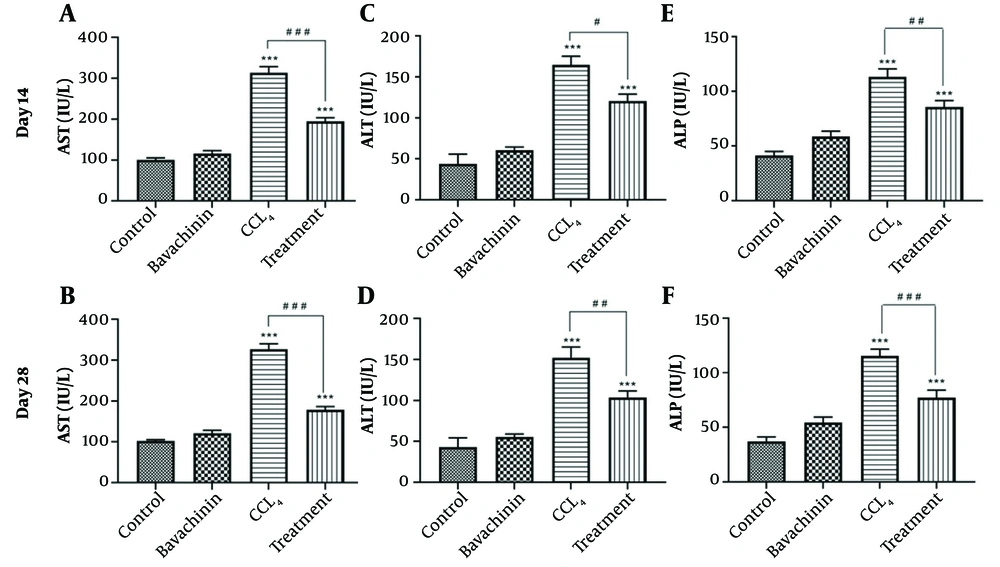
The analysis of alanine aminotransferase (ALT) (Figure 1C and D), aspartate aminotransferase (AST) (Figure 1A and B), and alkaline phosphatase (ALP) (Figure 1E and F) enzyme activities on days 14 and 28 illustrates the effect of BVC on ALT, AST, and ALP levels in serum (Table 1). Hepatotoxicity was confirmed by a significant increase in the activities of all three enzymes in the CCl4-treated and treatment groups compared to the control group (***P < 0.001). Treatment with BVC significantly reduced the release of these enzymes in serum on both days 14 and 28 compared to the CCl4-treated groups (#P < 0.05, ##P < 0.01, ###P < 0.001). It is evident that BVC had a more pronounced effect on decreasing AST levels than on ALT and ALP. Additionally, the reductions in AST, ALT, ALP, and MDA levels were more substantial on day 28. Furthermore, it was observed that the injection of BVC alone caused a slight increase in the levels of the test variables, but these changes were not statistically significant.
The effects of BVC on CCl4 -induced hepatotoxicity evaluated by aspartate aminotransferase (AST) (A and B); alanine aminotransferase (ALT) (C and D); and alkaline phosphatase (ALP) (E and F); activities in the serum. The results represent the mean ± SEM of seven animals per group. * Significantly different from the control group (***P < 0.001); # Significantly different from the CCl4- treated group (# P < 0.05) (## P < 0.01) (### P < 0.001).
| Group and Parameter | Day | Control | BVC | CCL4 | Treatment |
|---|---|---|---|---|---|
| AST (U/L) | 14 | 100.42 ± 4.96 | 116.28 ± 6.85 | 313.85 ± 14.57 *** | 194.42 ± 8.96 ***, ### |
| 28 | 102 ± 3.24 | 121.14 ± 7.10 | 326.57 ± 13.51*** | 178.57 ± 7.93 ***, ### | |
| ALT (U/L) | 14 | 43.71 ± 11.99 | 60.42 ± 3.97 | 164.57 ± 10.48 *** | 120.71 ± 8.17 ***, # |
| 28 | 43.28 ± 11.12 | 55.57 ± 3.57 | 152.14 ± 13.05 *** | 103.71 ± 7.91 ***, ## | |
| ALP (U/L) | 14 | 41.42 ± 3.63 | 58.57 ± 5.00 | 113.28 ± 7.31 *** | 85.71 ± 5.78 ***, ## |
| 28 | 37.14 ± 3.98 | 54.28 ± 5.15 | 115.42 ± 6.09 *** | 77.14 ± 6.88 ***, ### | |
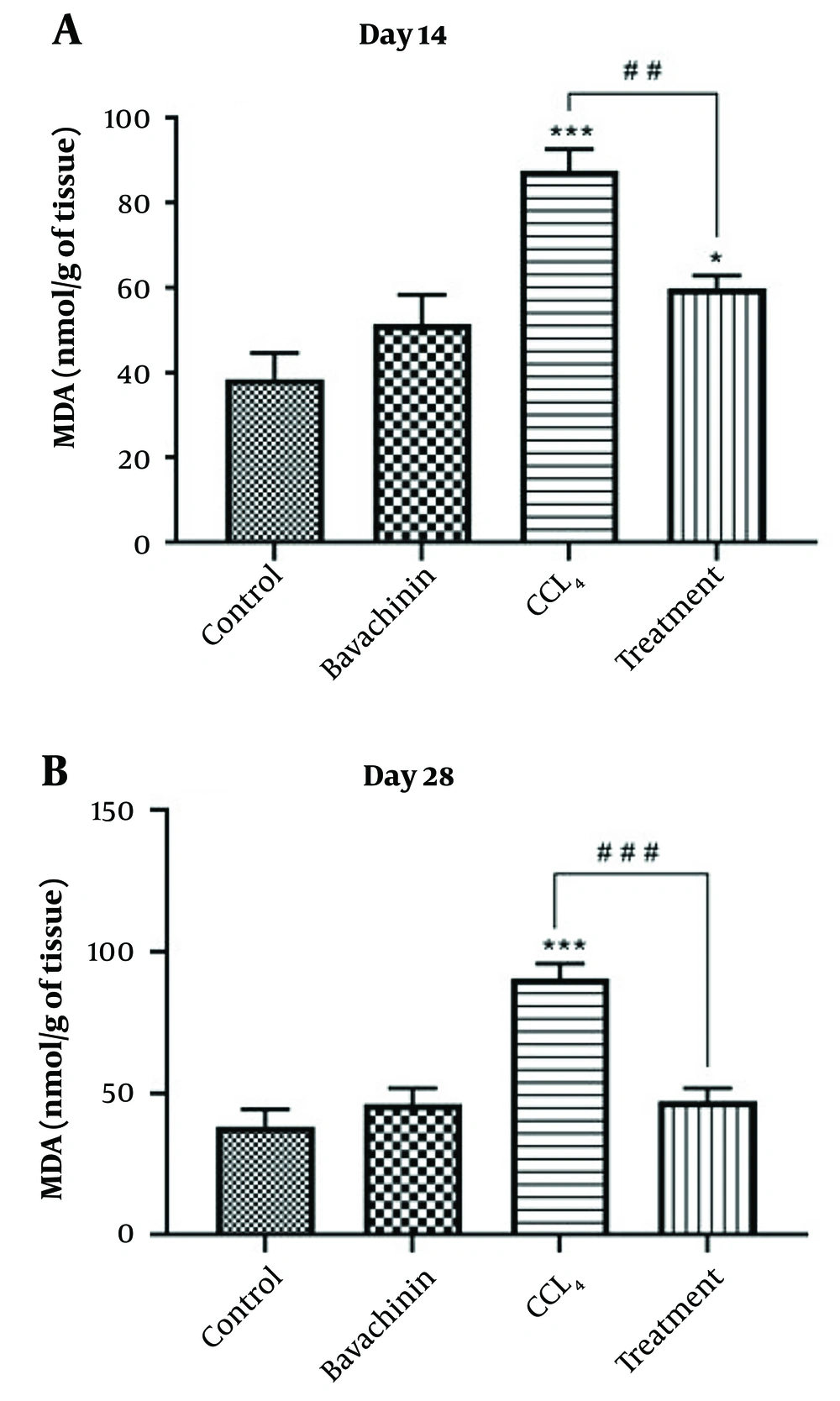
| MDA (nmol/mg protein) | 14 | 38.57 ± 6.03 | 51.42 ± 6.83 | 87.57 ± 4.94 *** | 59.85 ± 2.94 *, ## |
| 28 | 38.28 ± 5.99 | 46.14 ± 5.61 | 90.57 ± 5.27 *** | 47.14 ± 4.64 ### |
a The results represent the mean ± SEM of seven animals per group.
b Significantly different from the control group (* P < 0.1) (*** P < 0.001).
c Significantly different from the CCl4-treated group (# P < 0.05) (## P < 0.01) (### P < 0.001)
The amount of MDA, an end product of lipid peroxidation, was significantly higher in the CCl4-treated group compared to the control group on both day 14 and day 28 (P < 0.001). However, the MDA level in the treatment group was only significantly higher than the control group on day 14 (P < 0.05) (Figure 2A and B) (Table 1). The findings indicate that treatment with BVC significantly reduced the production of MDA in the liver compared to rats administered CCl4 alone. Specifically, BVC treatment resulted in significantly lower MDA levels on both day 14 (##P < 0.01) and day 28 (###P < 0.001), suggesting that BVC effectively mitigates oxidative stress induced by CCl4. Consequently, the activity of this oxidative stress marker in the BVC treatment group was significantly lower than in the CCl4 group, nearly reaching the levels observed in the control group. This implies that BVC has a strong protective effect against liver damage by reducing oxidative stress and lipid peroxidation, thus helping to maintain liver function closer to normal levels.
The effects of Bavachinin (BVC) on CCl4 -induced hepatotoxicity evaluated by malondialdehyde (MDA) generation in rat liver homogenate. The results represent the mean ± SEM of six animals per group. *Significantly different from the control group (* P < 0.05, *** P < 0.001). # Significantly different from the CCl4-only group (## P < 0.01, ### P < 0.001).
5. Discussion
In this study, we evaluated the hepatoprotective effects of BVC against CCl4-induced hepatotoxicity in adult male rats. The results demonstrated that BVC reduced hepatotoxicity caused by CCl4. Bavachinin treatment protected liver cells from CCl4-induced damage by lowering liver enzyme levels (ALT, AST, and ALP) and reducing lipid peroxidation (MDA). Moreover, significant hepatotoxicity was observed, as evidenced by increased levels of hepatic enzymes and MDA in rats treated with CCl4. These findings suggest that BVC has strong hepatoprotective effects by mitigating oxidative stress and preserving liver function, which aligns with previous studies on natural compounds and herbal extracts known for their liver-protective properties.
For example, Wang et al. investigated the hepatoprotective properties of Penthorum chinense Pursh against CCl4-induced acute liver injury in mice, highlighting significant reductions in liver enzymes and oxidative stress markers, similar to our findings with BVC (25). Another study by Ekpo et al. explored the flavonoid-rich fraction of Lasianthera africana leaves in alleviating CCl4-induced hepatotoxicity in Wistar rats, demonstrating comparable reductions in liver enzymes and oxidative stress parameters (26). Likewise, Sinaga et al. examined the hepatoprotective effect of Pandanus odoratissimus seed extracts against paracetamol-induced liver injury in rats, showing similar biochemical improvements indicative of liver protection (27).
Furthermore, studies focusing on specific compounds like BVC have also elucidated mechanisms of action relevant to our findings. For example, pharmacokinetic and metabolism studies of BVC have highlighted its bioavailability and potential therapeutic applications, supporting its role as an antioxidant and anti-inflammatory agent (28). Additionally, research into Psoralea corylifolia L., from which BVC is derived, has underscored its traditional use in Chinese medicine for treating liver disorders, reinforcing the rationale for studying BVC for hepatoprotection (29).
CCl4 toxicity is a well-established model of liver damage that can lead to liver lesions, cirrhosis, and hepatocarcinoma (30, 31). A clear sign of hepatic injury is the leakage of cellular enzymes into the plasma, which indicates cellular leakage and the degradation of the functional integrity of liver cell membranes (32). Drug-induced liver injury is associated with increased levels of AST, ALT, and ALP (33). During acute liver damage, large amounts of these enzymes are released into the bloodstream from the cytoplasm (ALT) and mitochondria (AST) of injured hepatocytes (34). Elevated serum ALP typically indicates injury to the canalicular membrane or biliary epithelial cells (35). Lipid peroxidation caused by CCl4 damages liver cell and organelle membranes, leading to the release of hepatic enzymes into the bloodstream (36). Consistent with these findings, our results showed that CCl4 treatment significantly increased the serum activities of ALT, AST, and ALP. Additionally, BVC inhibited the CCl4-induced increase in these enzyme levels and protected the liver from CCl4-induced damage in vivo. This demonstrates the stabilization of the plasma membrane and healing of CCl4-induced liver tissue injury and biliary dysfunction. These findings support the widely accepted view that hepatic parenchymal repair and hepatocyte regeneration, which increase the liver's resistance to toxin damage, cause the serum transaminase level to return to normal (32, 37).
The hepatotoxic effects of CCl4 are primarily due to its active metabolite, the trichloromethyl radical, which is converted to the trichloromethyl peroxyl radical (CCl3OO) in the presence of oxygen. These free radicals covalently bind to macromolecules, leading to the peroxidative degradation of membrane lipids, which are rich in polyunsaturated fatty acids (38). This process results in the formation of lipid peroxides that generate products like MDA, causing membrane damage (39, 40). The elevated levels of MDA in the liver of CCl4-treated rats suggest that the natural antioxidant defense system was compromised, impairing its ability to remove excessive free radicals (41). However, pretreatment with BVC significantly reduced MDA production, indicating hepatoprotection by preventing the initiation and progression of peroxidative processes in vivo.
Bavachinin is a flavonoid isolated from Psoralea corylifolia, one of the most widely used traditional Chinese medicines, derived from the dried fruit of P. corylifolia L. (18). It has been shown that BVC protects plasma membranes against various oxidative stresses (17). The biological activities of flavonoids, including free radical scavenging and antioxidant activities, are believed to be positively related to their chemical structures. These structures include the presence and arrangement of several functional groups, such as a catechol structure on the B-ring, a C2-C3 double bond together with a C4-ketone group, hydroxyl groups at C3 and C5 on the C and A rings, and glycoside groups at C6 and C8 on the A ring (42, 43).
The study on BVC and its hepatoprotective effects against CCl4-induced liver toxicity in male Wistar rats provides valuable insights, but several limitations should be considered. The use of only male Wistar rats limits the generalizability of the findings to broader populations, including potential gender-specific responses. Although the dosing regimen was clearly stated, a more detailed discussion on the rationale behind the chosen doses of BVC and CCl4 would help clarify the dose-response relationships. The study also offered limited mechanistic insights into BVC's protective mechanisms beyond biochemical markers. Additionally, the four-week duration of the study may not fully capture the long-term effects of chronic liver conditions induced by prolonged CCl4 exposure. Increasing the sample size and addressing potential reporting biases would also strengthen the study’s conclusions. Future research that addresses these limitations could provide a deeper mechanistic understanding and validate BVC's therapeutic potential for liver protection in diverse contexts.
In conclusion, BVC demonstrated protective effects against CCl4-induced hepatotoxicity in male Wistar albino rats, likely due to its antioxidant properties. However, further studies are needed to identify the molecular mechanisms responsible for its hepatoprotective effects.