1. Introduction
Ophthalmoplegia, defined as the paralysis of the eye muscles, can be caused by viruses and is categorized into external and internal subtypes (1). Varicella-zoster, the virus responsible for childhood chickenpox, lies dormant in the ophthalmic branch of the trigeminal ganglion (CN V1) and can reactivate to cause herpes zoster ophthalmicus (HZO) (2, 3). Herpes zoster ophthalmicus occurs in 10 to 25 percent of herpes zoster cases (4, 5). While most people assume that herpes zoster (HZ) primarily affects the elderly, young individuals with immune-compromised conditions are also susceptible, in addition to those with other risk factors such as age, ethnicity, and family history (6). Changes in the host immune system, particularly in cases of HZO, are a major factor in the development of HZ (4, 7, 8).
Herpes zoster symptoms include fatigue, fever, malaise, and vesicular rash, along with other constitutional symptoms. Ophthalmoplegia can develop weeks after the acute phase of the disease, leading to both acute and chronic ocular problems (9). Typical signs of ocular involvement include conjunctivitis, iridocyclitis, glaucoma, retinitis, keratitis, and uveitis, often accompanied by increased intraocular pressure (10, 11). Although ocular nerve palsies are uncommon, a few case studies have documented isolated palsies of the oculomotor, trochlear, and abducens nerves associated with limitations in ocular motility (1, 12, 13).
Here, we describe a rare instance of HZO that manifested as an acute anisocoric nonreactive pupil, ocular movement limitation, and blurry vision. Following treatment, the condition resolved completely over four months without further complications, such as keratitis, uveitis, or conjunctivitis.
2. Case Presentation
A 37-year-old female patient with no previous medical history presented to our hospital with major complaints of acute drooping of the right upper eyelid, impaired vision, and limited eye movements. She had a history of insect bites. A dermatologist had suggested some topical ointment treatments 10 days before these symptoms appeared. She initially complained of soreness around her upper right eyelid, but there was no clear indication of vesicular lesions. By the time of admission, tiny vesicular and crusty lesions with a limited extension around the right eye canthus and lateral wall of the nose had formed. After four days, multiple vesicles appeared on the right upper eyelid and forehead, without involving the eyes. One week after the skin manifestations, the patient developed acute ptosis of the right eye along with hazy vision. She denied experiencing diplopia or a decrease in her right eye's vision.
An ophthalmologist consultation revealed that the best-corrected visual acuity was 8/10 in the right eye and 10/10 in the left eye. Upon examining the right eye, noticeable ptosis was observed, along with healed crusted scars on the right side of the forehead and eyelid, as well as swelling in the right orbit. The left eye's confrontation visual fields were full enough to count fingers, but a minor superior field deficiency in the right eye was caused by right eyelid ptosis and significant edema. There was no evidence of circumcorneal congestion, keratitis, or proptosis. Corneal sensations were present in both eyes, but the right pupil was dilated and unresponsive to direct consensual light. Extraocular movements of the right eye were limited to downward, inward, and upward gaze, while the left eye movements were normal. Ophthalmoscopy examination of the disc and macula on both sides was normal. The right pupil dilation during the consensual light response was accompanied by a reverse relative afferent pupillary defect (RAPD), indicating a relative afferent pupillary defect in the right eye.
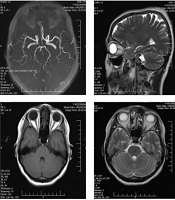
The dermatologist, who had seen the patient for vesicular lesions, agreed with the ophthalmologist that the patient's third nerve palsy was caused by herpes zoster ophthalmicus. To exclude other possible causes of third nerve palsy, brain magnetic resonance imaging (BMRI) and brain magnetic resonance angiography (BMRA) examinations (Figure 1) were performed, and the results were normal. A course of treatment was initiated involving oral prednisolone (50 mg/day) and intravenous acyclovir (400 mg three times a day). After seven days of treatment, the patient was discharged with oral acyclovir at the same dosage (800 mg five times a day) and a three-week tapering dose of prednisolone. Topical eye treatments, including erythromycin ointment and artificial tears, were also continued.
2.1. Follow Up
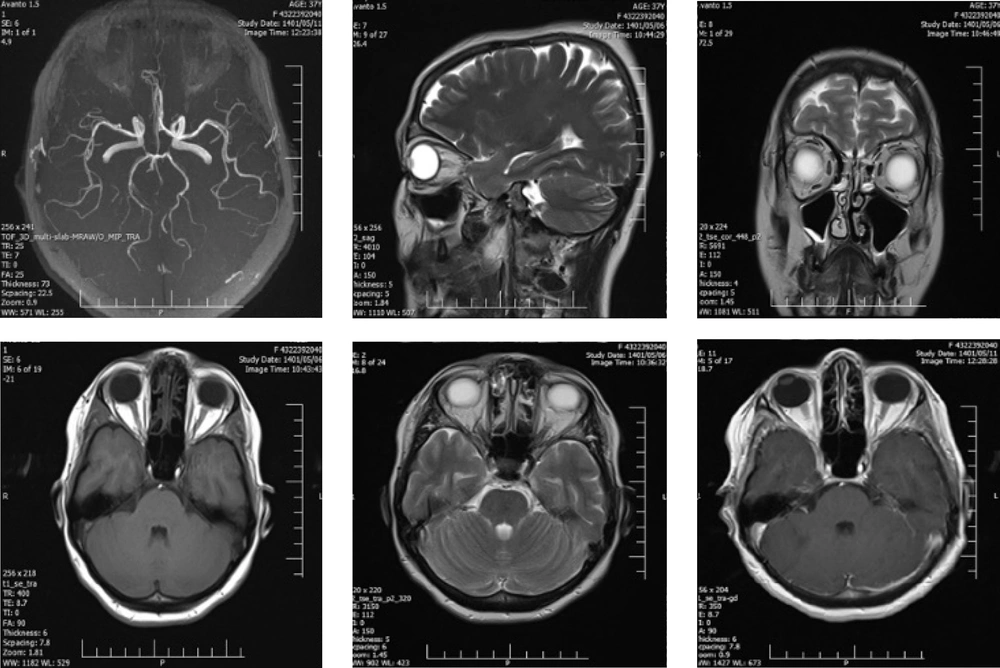
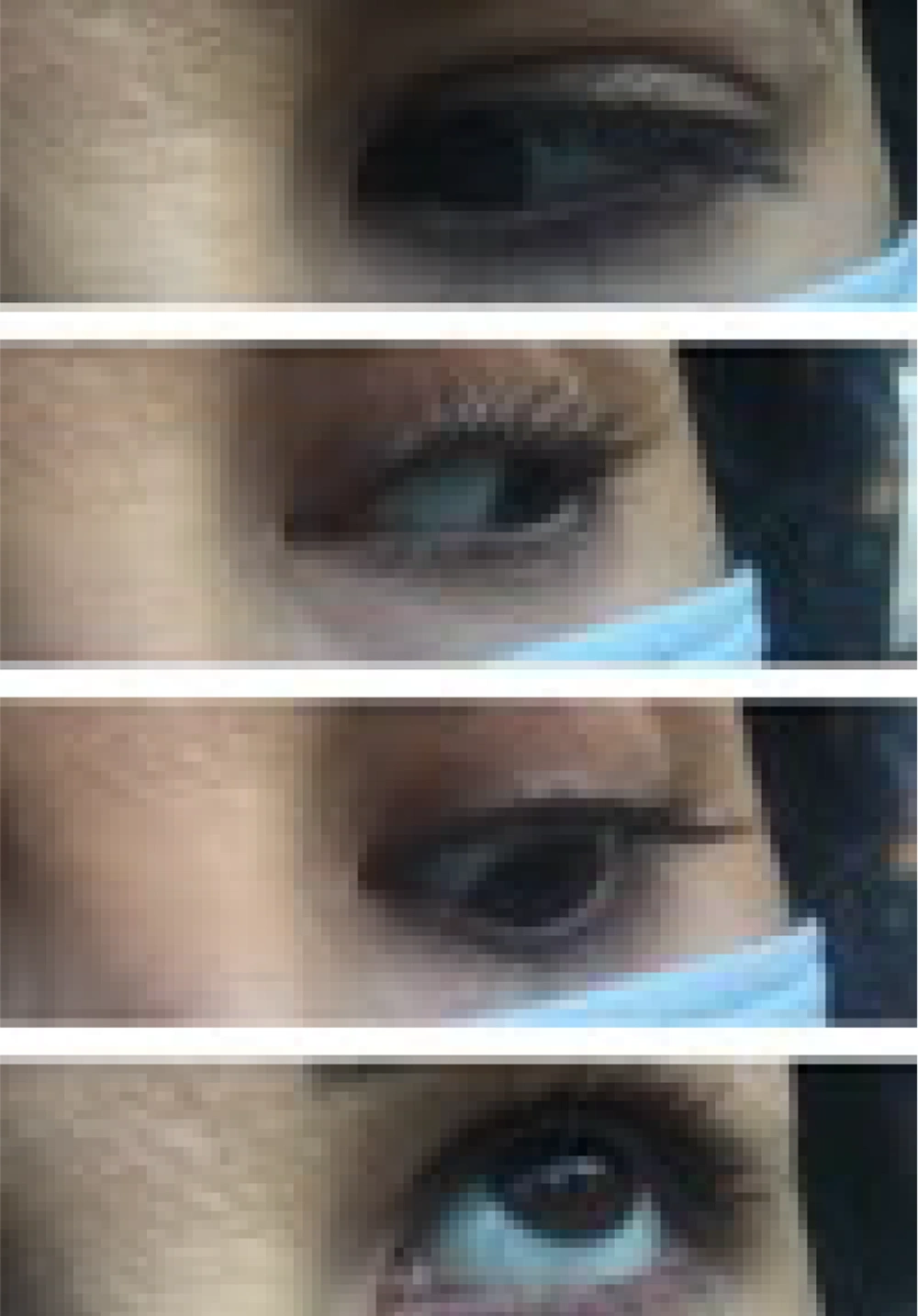
Her follow-up period lasted four months. At the time of discharge, she had blurred vision and a non-reactive mydriatic pupil in the affected eye, despite slight improvement in ptosis and ocular motility (Figure 2).
Two weeks later, the patient reported that her ptosis and eye movements had not improved. A pupil examination revealed a slight reaction to consensual light in the right pupil and reduced ptosis in the right eye. However, there was no noticeable improvement in ocular motility.
After one month, the drooping of her right eyelid had decreased, and her visual acuity was normal. Compared to the left pupil, the right pupil's response to light was much less pronounced, but her ocular movement had significantly improved.
After two months, there was a minor decrease in the severity of her right ptosis, and she had better ocular movement in the nasal, superior, and inferior gaze. The patient noticed a significant improvement in her symptoms.
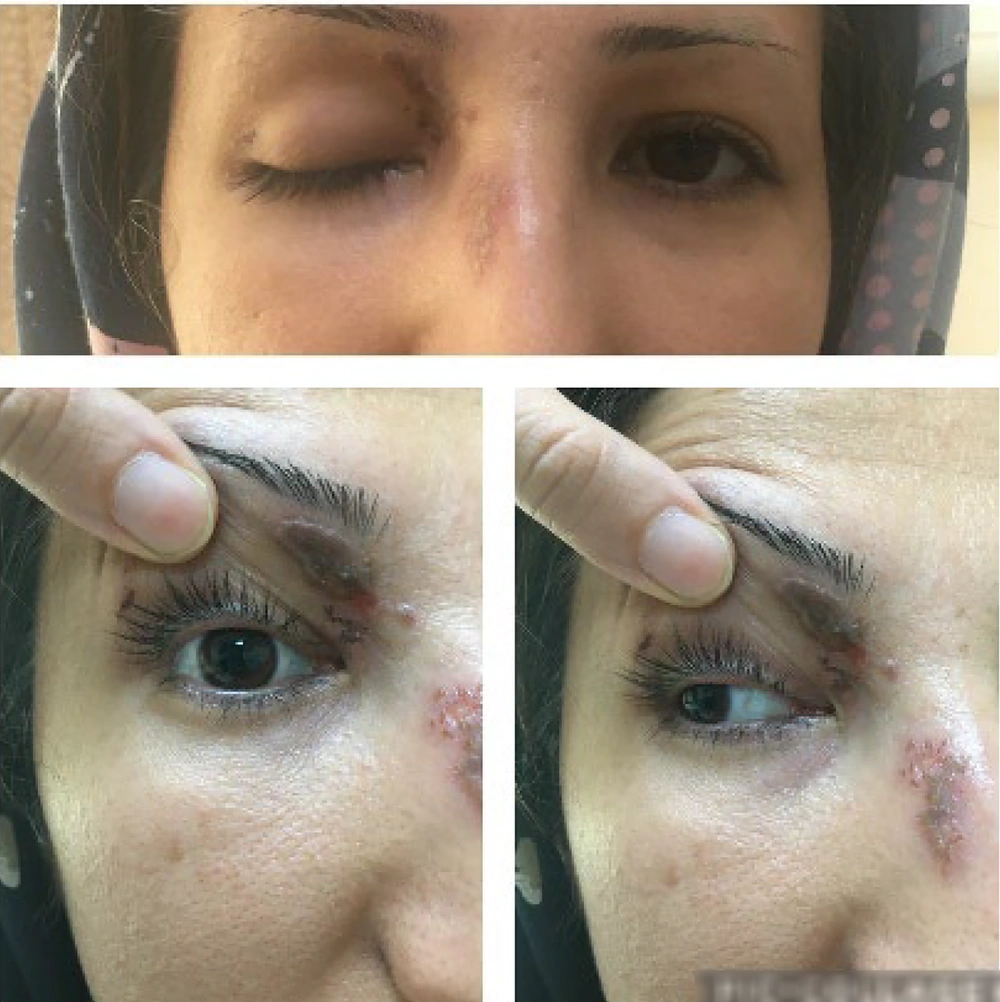
Three months after her initial presentation, her vision had fully returned to normal (Figure 3). Her confrontation visual fields were better than in previous visits. Although her right-side ocular movement remained somewhat limited, her pupil response appeared to be normal.
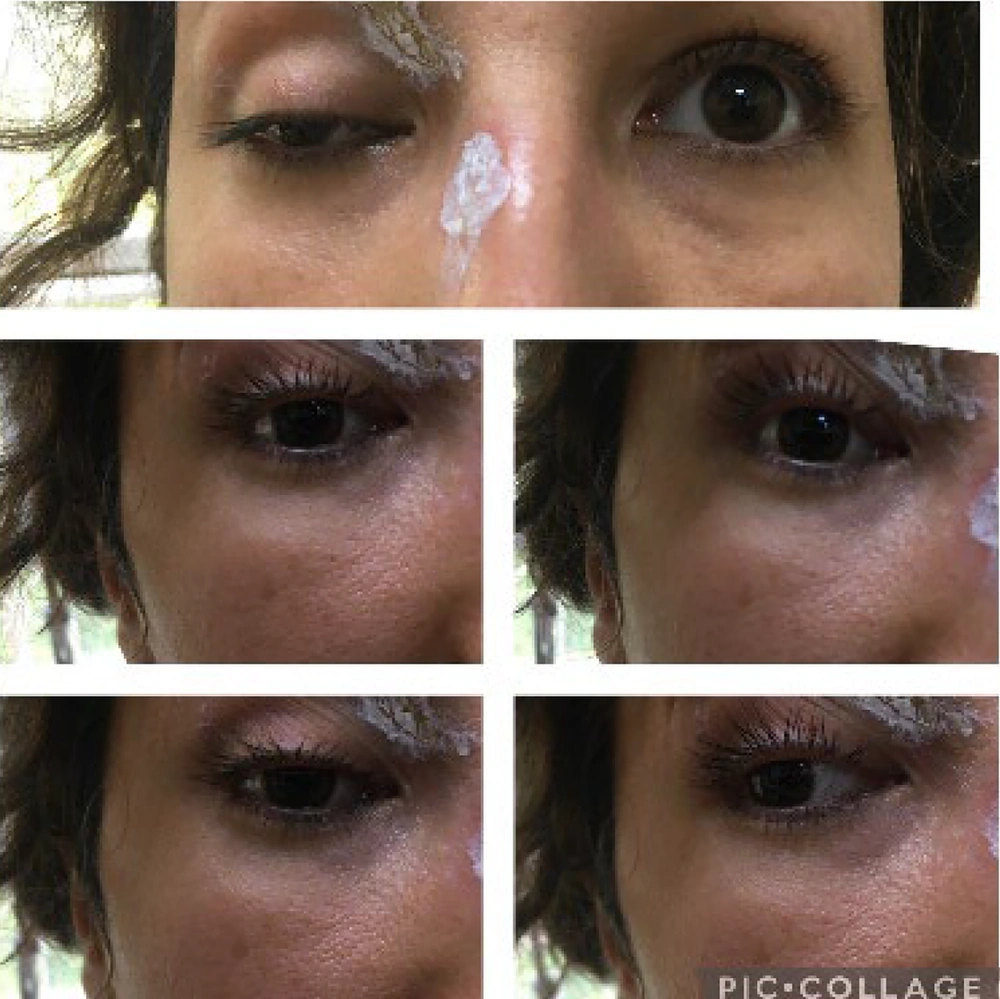
At the four-month follow-up, the patient had no complaints. She was able to move her eyes normally again, and her ptosis had considerably improved without affecting her field of vision (Figure 4). Follow-up visits were scheduled three months apart.
3. Discussion and Conclusion
Herpes zoster ophthalmicus is the reactivation of latent varicella-zoster virus associated with a vesicular rash or dermatitis in a specific zone, as well as in the ocular division of the V1 cranial nerve (6, 13). The rare extra-cutaneous signs include neurological, ophthalmic, hepatic, and pulmonary issues. About 50% of patients with HZO may experience ocular problems such as conjunctivitis, episcleritis, dendritic keratitis, anterior uveitis, or retinitis (1, 10, 14). Ten percent of all HZ cases involve HZO-associated full ocular paralysis, a rare condition that results in ophthalmoplegia in 11 - 29% of cases due to HZ reactivation (15). Ocular paralysis can lead to meningitis, cranial neuropathy, muscular ischemia, and vasculitis (5, 16, 17).
Third nerve palsy is the most common type of palsy in HZO, followed by CN VI and CN IV palsies, respectively (1, 5, 18). Herpes zoster ophthalmicus primarily affects the elderly population, often presenting with more severe symptoms than in younger individuals (1). In this case, however, our patient was a young person with significant symptoms, including vision abnormalities and complete third nerve palsy. According to statistical data, 75% of cases of HZO with ophthalmoplegia occur 9.5 days after the rash first appears (15). In our case, however, ophthalmoplegia manifested abruptly with full features as the first symptom following dermatitis.
Neuroimaging is required to rule out a compressive lesion in a patient with nerve palsies, whether pupil-involving or pupil-sparing (3). Neuroimaging investigations were conducted in our case, despite it being an atypical form of HZO with pupil involvement and third nerve palsy. The imaging results were not significant. Ophthalmoplegia is a self-limiting form of HZ and resolves completely (in 4.4 months) in 65 - 76.6% of patients without therapy, according to recent research (1, 15). Complete recovery has been found to take between one and eighteen months based on case series research (3). In our patient, ophthalmoplegia fully disappeared four months after the vesicular rash started.
Most of the time, oral acyclovir and steroid therapy are the first steps in treatment; however, in this instance, acyclovir treatment began with IV administration, continued orally after seven days, and prednisolone was tapered over three weeks without any negative side effects. Overall, this case serves as an example of the unusual ophthalmoplegia brought on by HZO. It is crucial to control the symptoms, and prompt treatment can significantly lessen the severity of HZO symptoms.