1. Introduction
Kawasaki disease (KD) was first identified in Japan in 1967 and has since been globally recognized as a significant pediatric health issue. Characterized by acute inflammation leading to vasculitis, KD primarily affects the coronary arteries (1). Although the precise etiology remains unknown, an exaggerated immune response to an infectious agent is hypothesized, with genetic predisposition also playing a role (2). The KD is diagnosed clinically, adhering to established criteria requiring prolonged fever and specific clinical manifestations. Untreated KD can lead to coronary artery abnormalities in 20% to 25% of cases, while prompt administration of intravenous immunoglobulin (IVIG) reduces this risk to approximately 2% to 5% (3, 4).
2. Pathophysiology
The pathophysiology of KD involves an immune-mediated inflammatory response, wherein an unidentified pathogen triggers an exaggerated immune reaction in genetically predisposed individuals. This leads to the activation of T-cells, macrophages, and the production of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) (5). The inflammation predominantly affects medium-sized arteries, with the coronary arteries being the most critically impacted. This can result in coronary artery aneurysms, myocardial ischemia, and even myocardial infarction (6).
3. Diagnostic Criteria
The diagnostic criteria for classic KD include:
3.1. Fever Lasting Five or More Days
3.1.1. At Least Five of the Following Five Clinical Features
- Bilateral conjunctival injection
- Changes in the lips and oral cavity (e.g., cracked lips, strawberry tongue)
- Cervical lymphadenopathy (≥ 1 lymph node > 1.5 cm in diameter)
- Extremity changes (e.g., edema, erythema)
- Polymorphous rash
Incomplete KD is suspected when a patient presents with fever lasting at least five days and only two or three of the principal features (7, 8). Early echocardiographic evaluation is crucial for infants younger than six months with unexplained prolonged fever, as they are at increased risk for coronary artery involvement (9).
4. Case Presentation
4.1. Case 1
An 8-year-old boy with a history of KD treated with IVIG at the age of two presented with dyspnea on exertion and palpitations. He had previously experienced no cardiac complications. On examination, he exhibited significant tachycardia and mild respiratory distress.
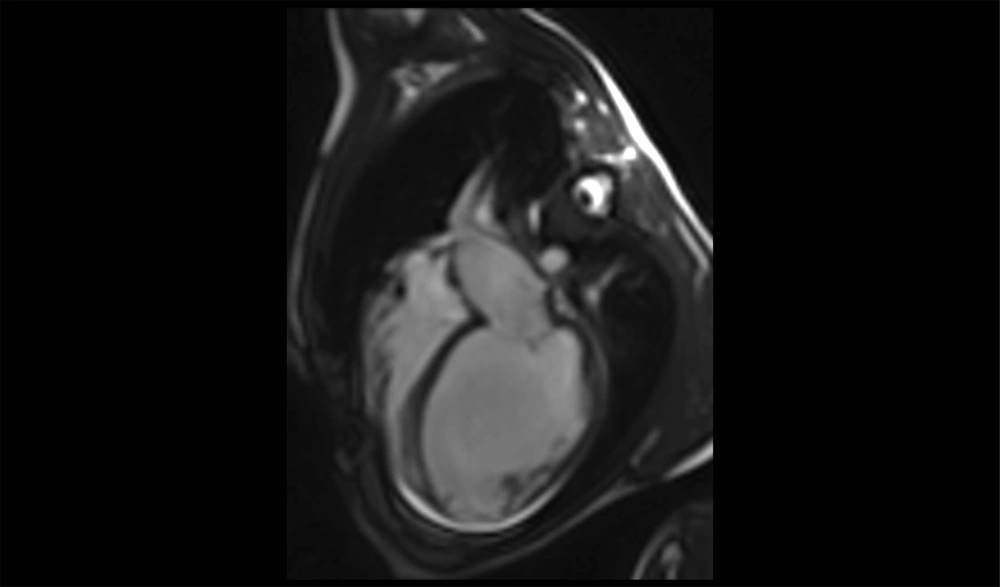
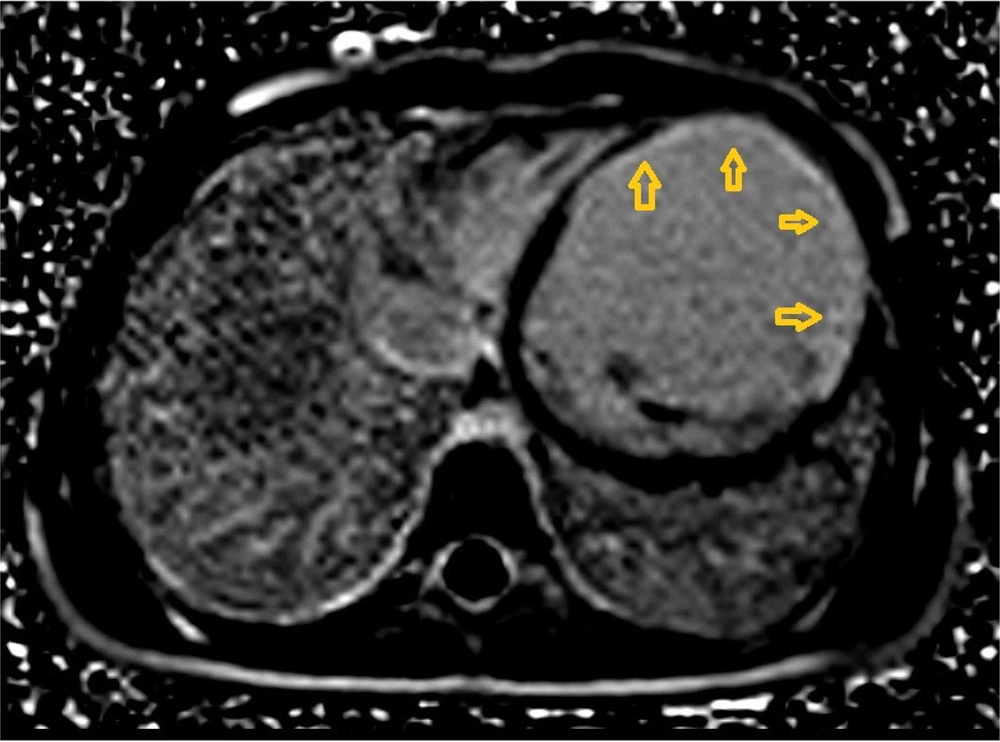
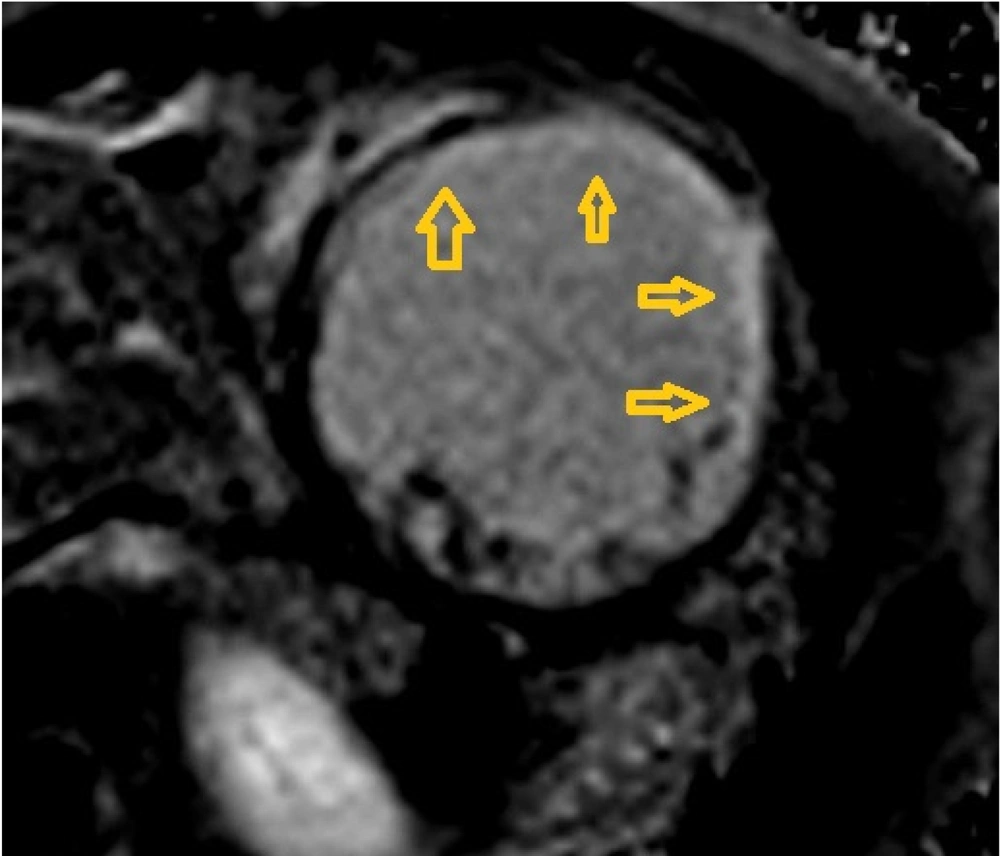
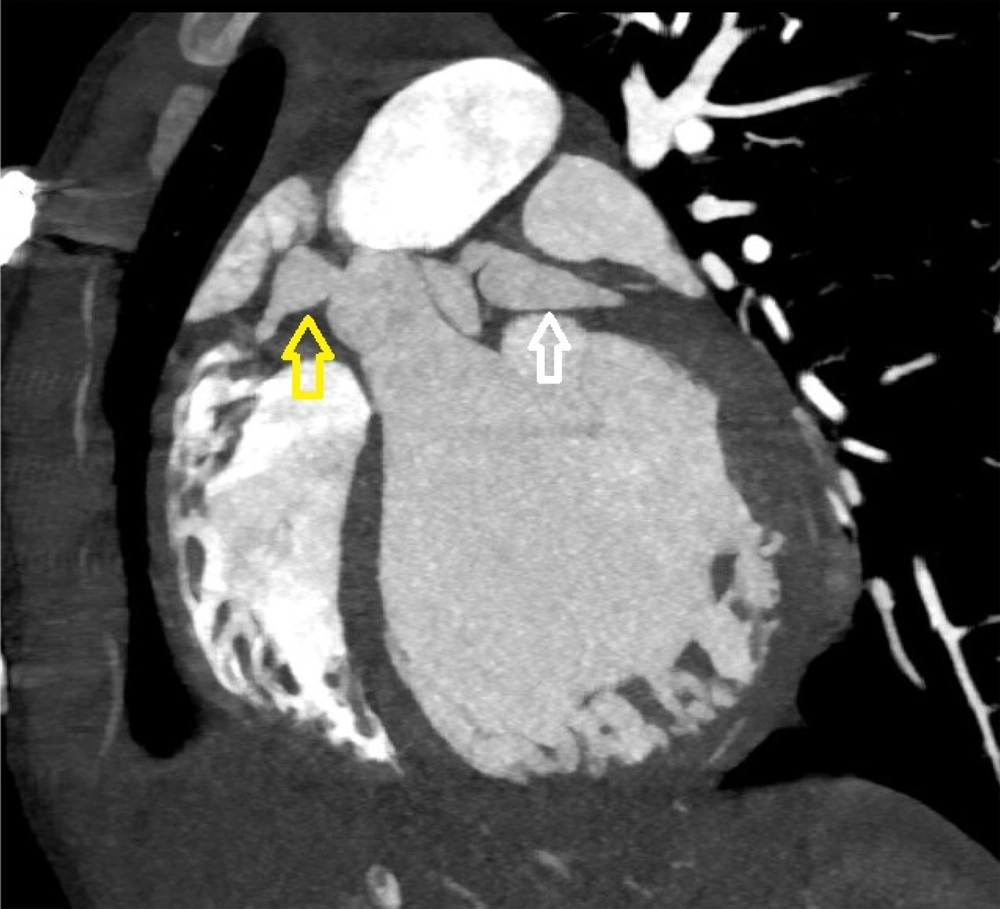
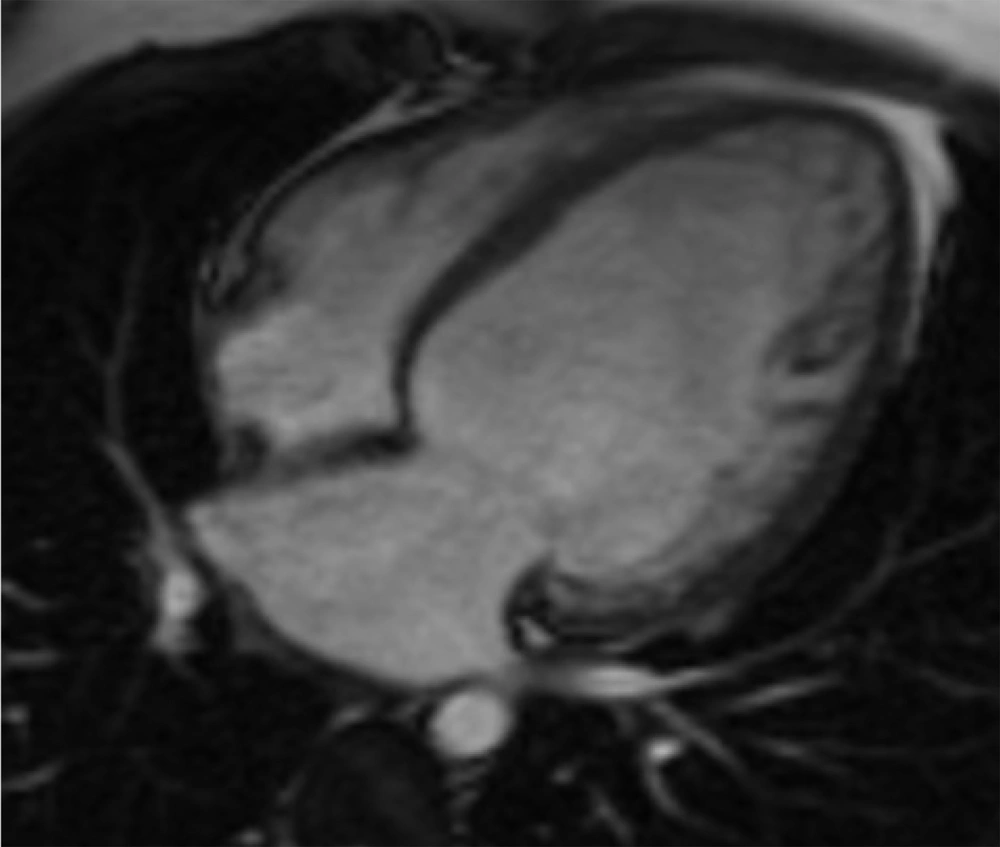
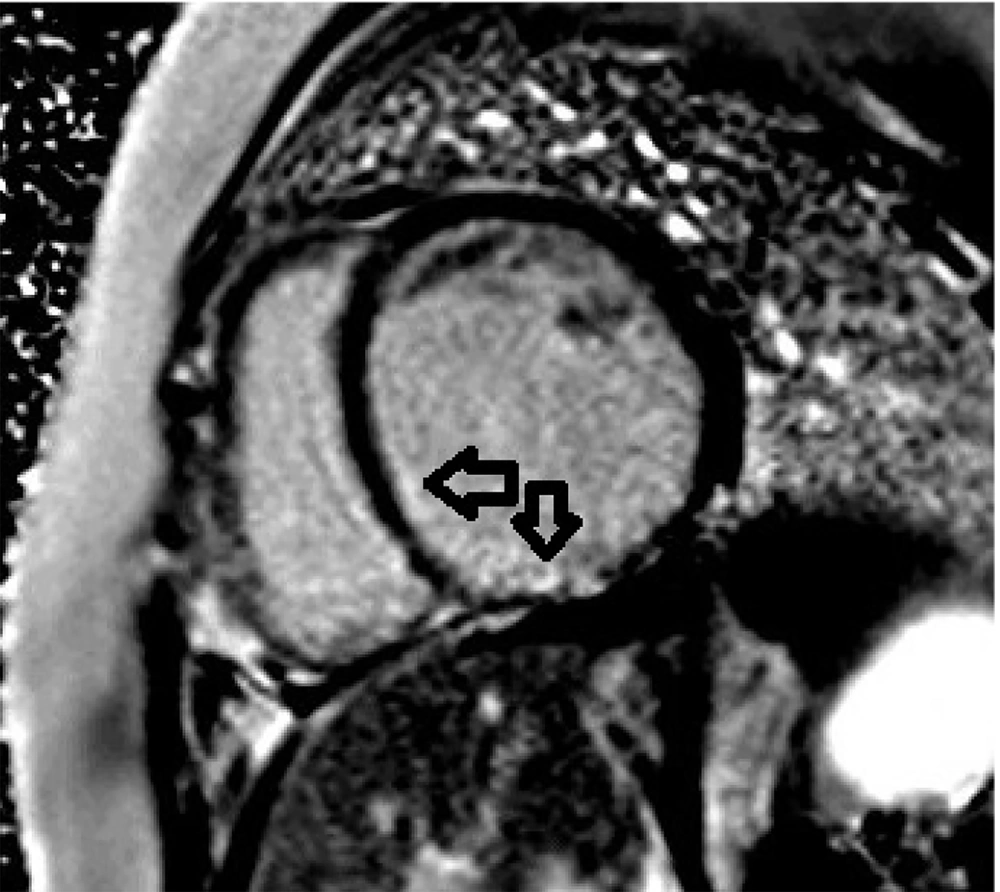
Initial echocardiography revealed severe left ventricular (LV) dilation (LV end-diastolic diameter of 60 mm) and significantly reduced systolic function (ejection fraction of 30%). Additionally, severe mitral regurgitation was observed. Cardiac magnetic resonance imaging (CMR) was performed to assess myocardial involvement, revealing severely reduced systolic function without left ventricular hypertrophy, regional wall motion abnormalities (RWMA) in the anterior and anteroseptal segments, and significant non-viable tissue in the left anterior descending (LAD) artery territory, indicating a previous myocardial infarction (Figures 1 - 3).
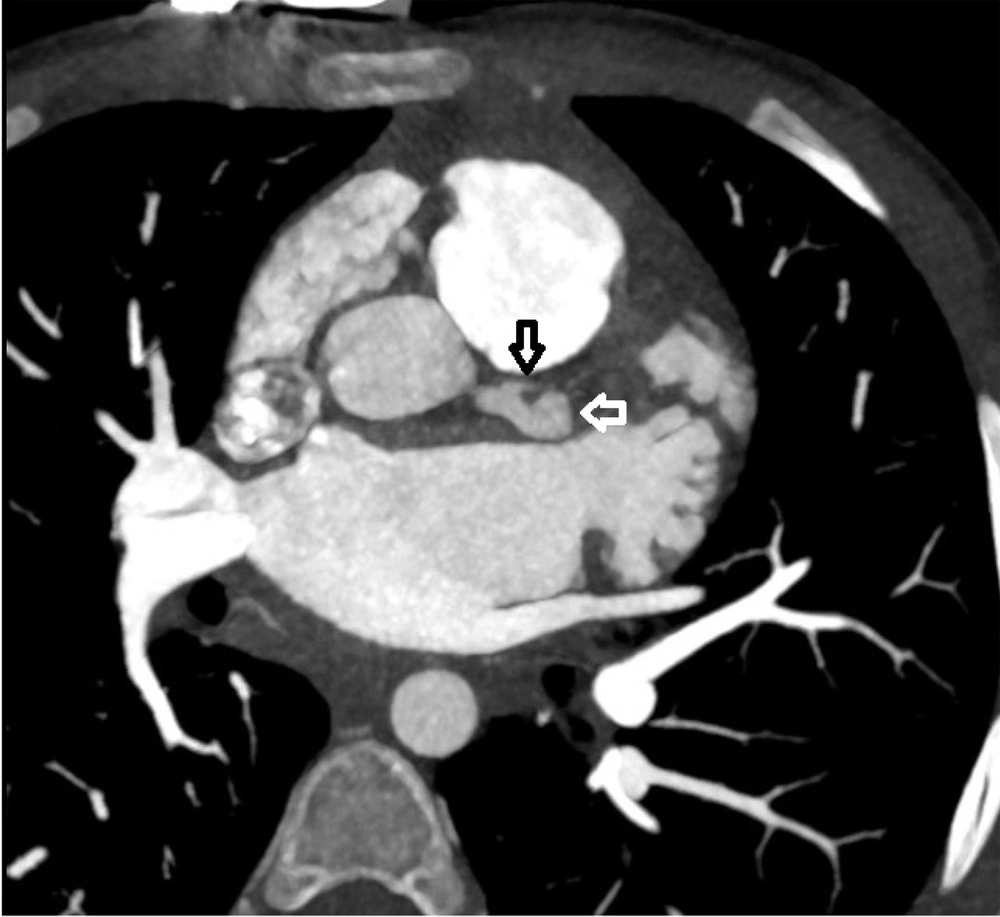
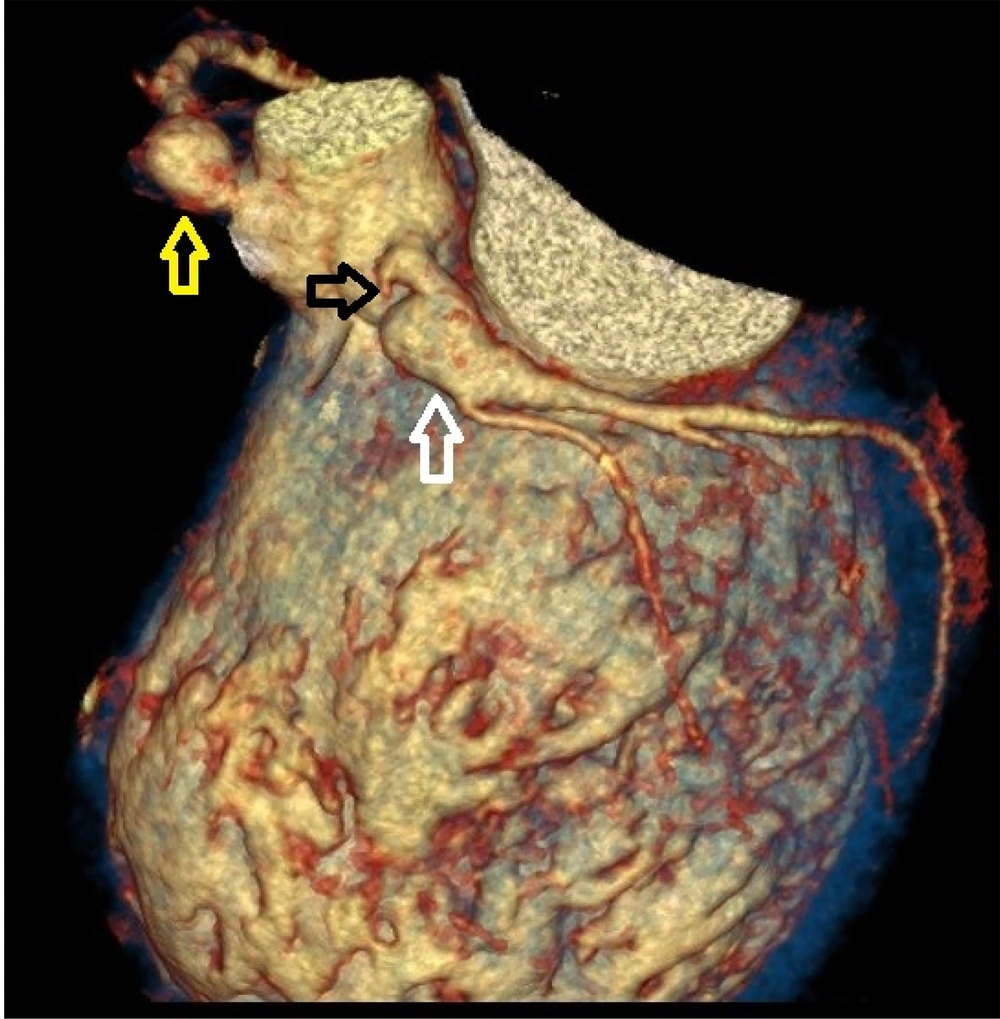
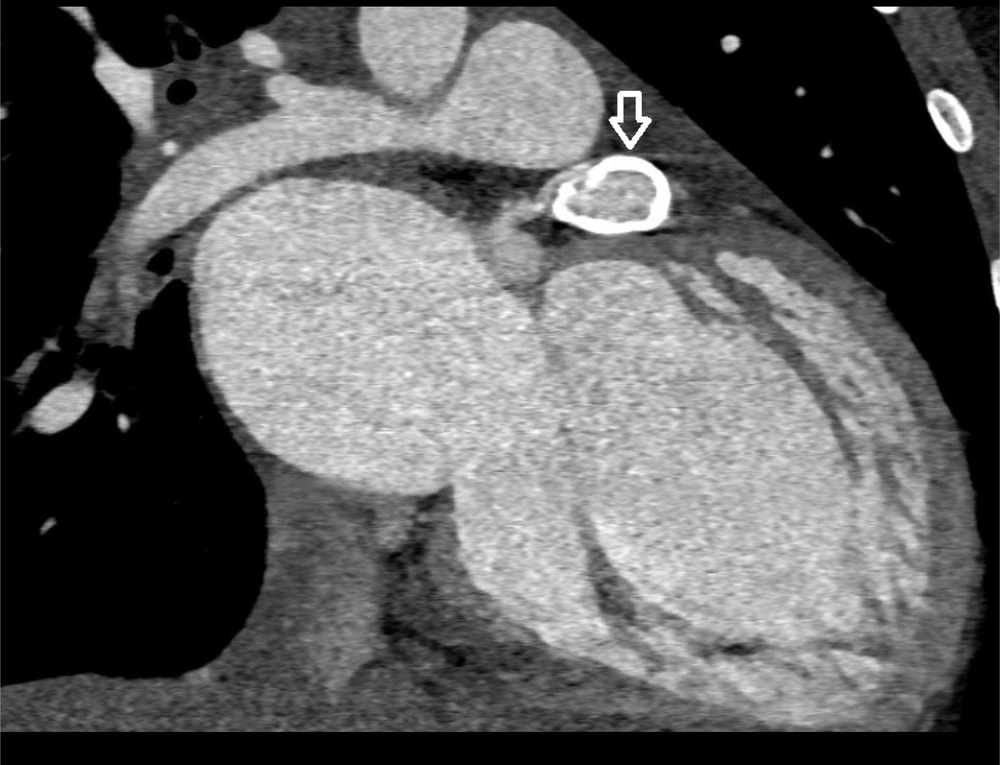
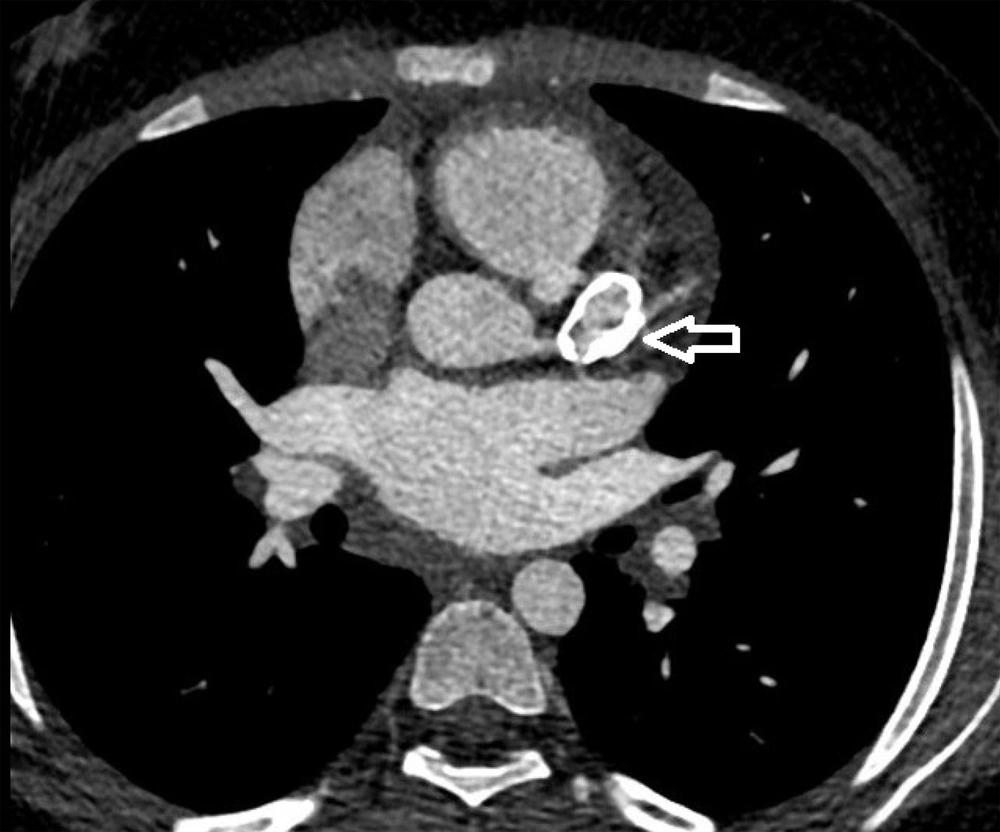
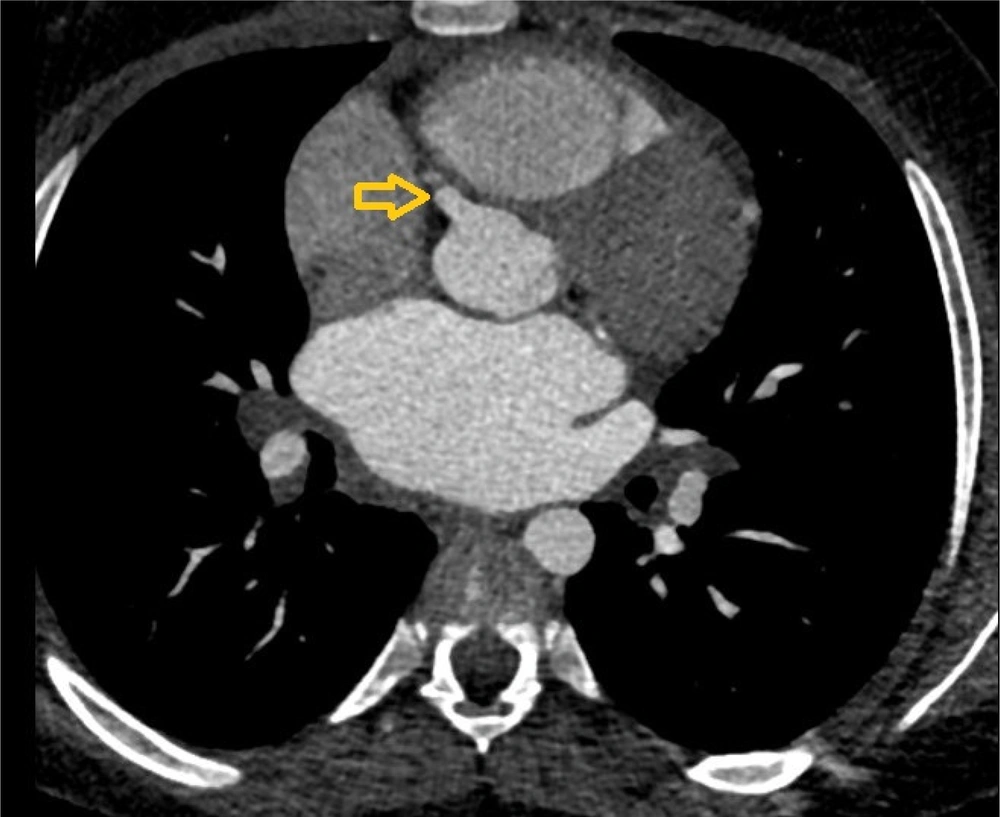
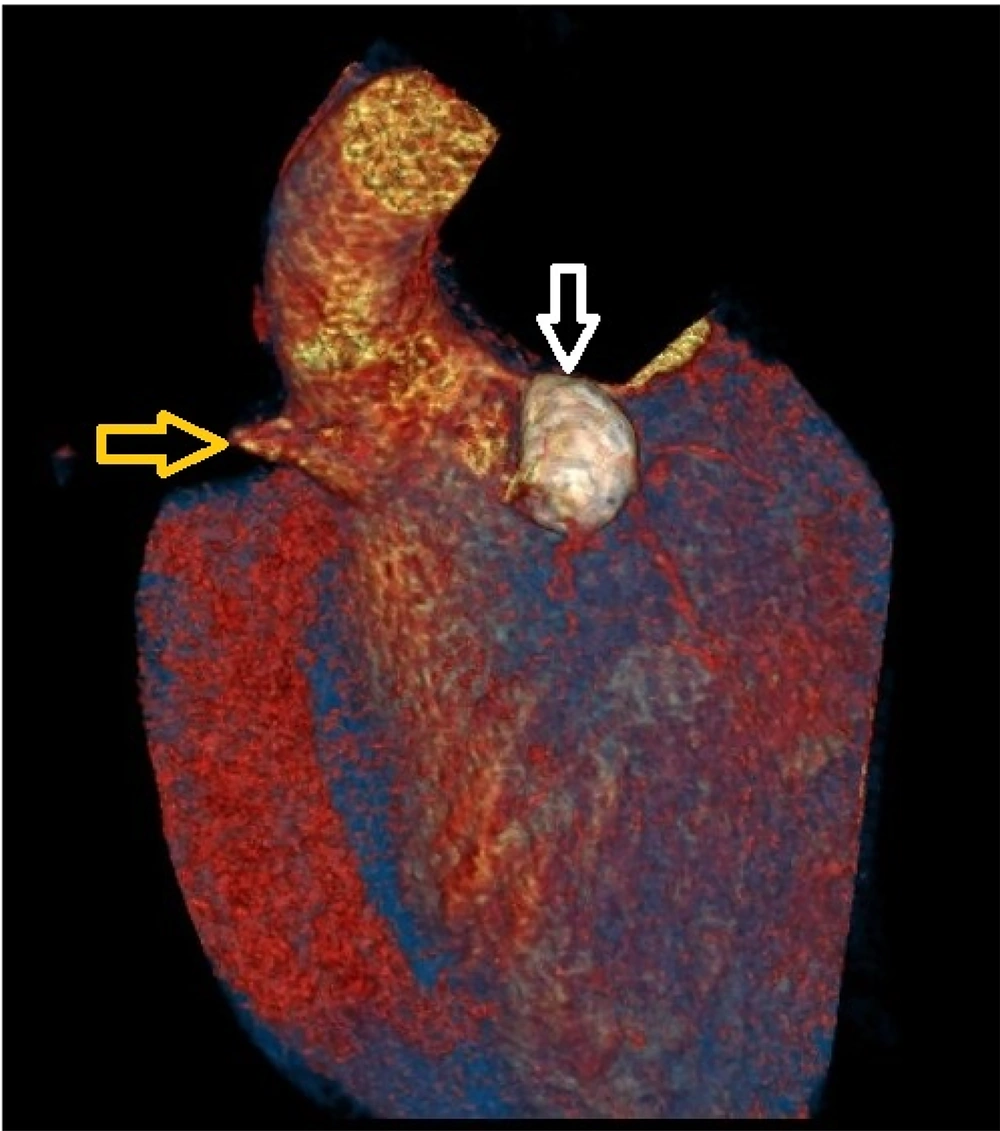
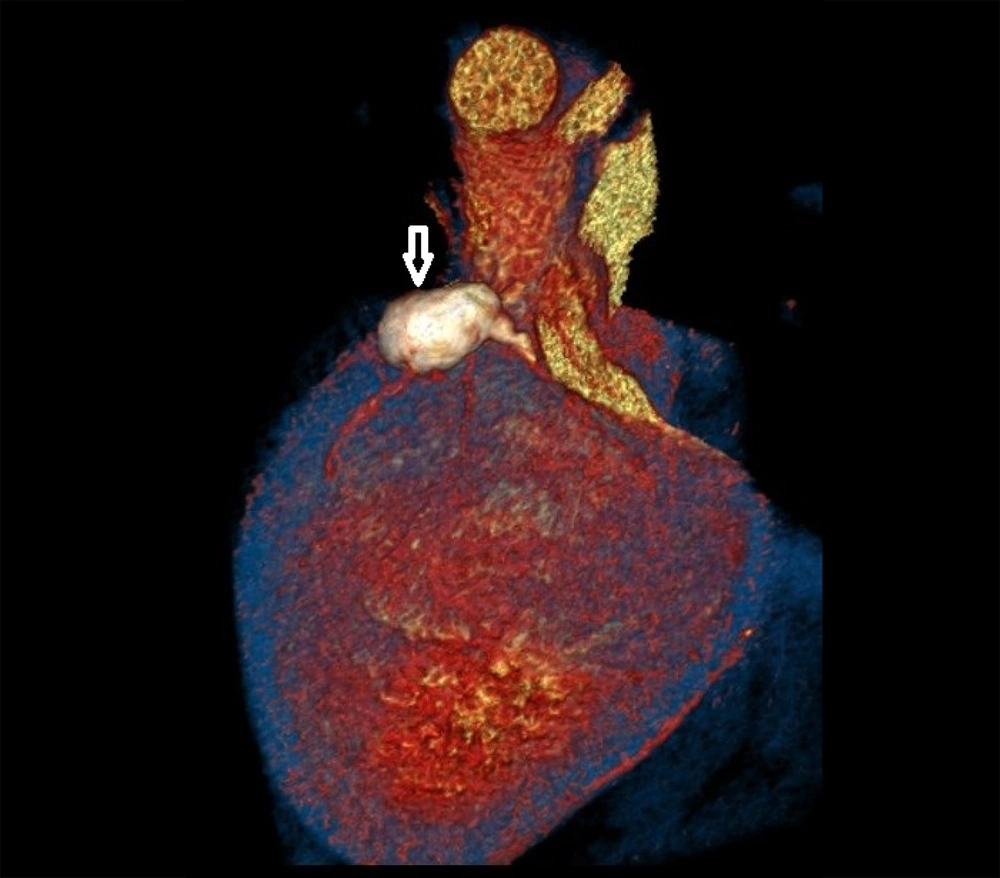
Coronary CT angiography showed two coronary aneurysms: One measuring 8 mm in the left circumflex artery and another 8.6 mm in the right coronary artery. Most concerning was the severe stenosis of the LAD, which was critically narrowed from its origin (Figures 3 - 6).
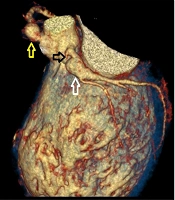
Given the severity of his condition and the absence of promising conservative or surgical treatment options, the child was evaluated for heart transplantation. He was placed on the waiting list and closely monitored in the pediatric intensive care unit (PICU), with an extracorporeal life support device on standby. After 82 days, he successfully underwent heart transplantation. The explanted heart exhibited severe coronary artery changes, with thick, fibrotic, and sclerotic vessels resembling "chicken feet," accompanied by proximal aneurysms.
Post-transplant, immunosuppressive therapy was initiated with prednisolone, mycophenolate mofetil, and tacrolimus, without induction therapy. Perioperative antibiotic prophylaxis included cefotaxime and vancomycin. The first endomyocardial biopsy, performed two weeks post-transplant, showed no signs of rejection (ISHLT grade 0R). The postoperative course was uneventful, and the patient was discharged on postoperative day 38. At discharge, he was prescribed immunosuppressive medications along with antiviral and antifungal agents for infection prevention. Follow-up visits indicated stable cardiac function with no signs of rejection.
4.2. Case 2
A 14-year-old boy diagnosed with KD 11 years earlier presented with dyspnea on exertion and palpitations. He had received inadequate treatment during the acute phase of KD. On examination, he demonstrated a bounding pulse and signs of heart failure.
Initial echocardiography revealed severe LV dilation (LV end-diastolic diameter of 65 mm) and significantly reduced systolic function (ejection fraction of 11%). Left atrial enlargement was also noted, with a smoky pattern in the left atrial cavity indicative of thrombus formation. The right ventricle was normal in size but displayed mild systolic dysfunction. The tricuspid annular plane systolic excursion (TAPSE) was 22 mm, with severe mitral regurgitation and moderate tricuspid regurgitation.
A 24-hour Holter monitor showed infrequent premature ventricular contractions and significant ST-T changes indicative of ischemia. Cardiac magnetic resonance imaging (CMR) confirmed severely reduced systolic function (ejection fraction of 11%) and regional wall motion abnormalities (RWMA), consistent with previous myocardial infarction (Figures 7 - 8).
Coronary CT angiography revealed two large aneurysms in the proximal left anterior descending (LAD) artery and right coronary artery territories, along with significant stenosis (Figures 9 - 13). Coronary angiography and right heart catheterization (RHC) confirmed a giant aneurysm in the left coronary artery, with elevated left ventricular pressure (90/20 - 30 mmHg).
Due to the lack of viable conservative or interventional treatment options, the child was evaluated for heart transplantation and placed on the waiting list. He was monitored closely in the PICU, with an extracorporeal life support device on standby. After 17 days, he successfully underwent heart transplantation. The explanted heart revealed severe coronary artery changes, characterized by thick, fibrotic, and sclerotic vessels, accompanied by proximal aneurysms.
Post-transplant, immunosuppressive therapy was initiated similarly to Case 1. The first endomyocardial biopsy, performed two weeks post-transplant, showed no signs of rejection (ISHLT grade 0R). The postoperative course was stable, and he was discharged on postoperative day 38. Follow-up assessments indicated stable cardiac function, with no evidence of rejection.
5. Discussion
The management of KD is complex, particularly when coronary artery complications develop. These cases highlight the critical importance of early diagnosis and timely intervention in mitigating severe outcomes. Echocardiography remains the first-line imaging modality for detecting coronary aneurysms and other cardiac abnormalities associated with KD. If necessary, CT angiography serves as the gold standard for assessing coronary artery involvement (10, 11).
Despite aggressive treatment strategies, including IVIG and corticosteroids, some patients progress to end-stage heart failure due to extensive coronary artery disease (12). Various catheter-based and surgical interventions, such as coronary artery bypass grafting (CABG) and percutaneous coronary interventions (PCI), may be employed in select cases; however, heart transplantation remains the last resort for patients with severe coronary involvement (13).
The first successful cardiac transplantation in a KD patient was reported in 1991, underscoring the need for continued advancements in transplantation medicine (14). Improvements in immunosuppressive therapy have further enhanced outcomes for these patients. Nevertheless, the complexities of managing chronic KD necessitate a multidisciplinary approach and a comprehensive understanding of the potential long-term cardiovascular consequences (15).
5.1. Conclusions
Early recognition and treatment of KD are crucial for reducing the risk of coronary artery involvement and subsequent complications. While most patients respond well to IVIG therapy, a subset may develop severe coronary artery abnormalities necessitating aggressive interventions, including heart transplantation. The complexities of managing chronic KD underscore the need for further research to enhance treatment strategies and improve patient outcomes.
.jpg)