1. Background
The causative agent of the infectious disease dengue is a positive-sense RNA virus from the Flaviviridae family, and it has five serotypes: 1, 2, 3, 4, and 5 (1, 2). Unlike the other four serotypes, which follow an anthropogenic cycle (with humans being the only known reservoir host), DENV-5 follows a sylvatic cycle. In this cycle, the virus is primarily transmitted among non-human primates, such as rhesus monkeys, which can infect humans. Dengue (DEN) sylvatic is endemic in natural forests in tropical and subtropical regions, including West Africa and the Malaysian Peninsula. In and around these forests, activities such as forest work and poor waste management systems create optimal conditions for the emergence of DENV-5, enabling mosquitoes to transmit the virus from monkeys to humans (2, 3).
In humans, dengue fever (DF) is considered one of the ten major global health threats and is one of the most important infectious diseases in tropical and subtropical regions. It is transmitted by arthropods and holds particular significance for public health (4-6).
Climate changes in human environments have significant and alarming effects on tropical infectious diseases. These climate changes are often regarded as a distinct factor in explaining the dynamics of the dangerous viral disease, DF. Some studies suggest that climatic factors should be considered one of the main determinants in the epidemiological complex, which includes vector ecology, pathogen biology, disease transmission, disease occurrence and prevalence, as well as disease control, prevention, and treatment (7, 8). Among these factors, the effects of key weather variables—such as temperature, relative humidity, rainfall, and seasonality—along with greenhouse gas emissions and human population density, play a significant role in the pathogenesis of DF (7, 9, 10).
Aedes aegypti and Aedes albopictus mosquitoes are carriers of DENV, and the virus is transmitted to humans through the bite of an infected female mosquito. Malaysia, India, and Pakistan are among the dengue-endemic countries, where an average of 75 million people contract DF every year (3).
By 2080, it is predicted that 60% of the world's population will be at risk of contracting the dengue virus (DENV) (11). Infection with this virus may remain asymptomatic or lead to a wide range of clinical manifestations (12).
Currently, there is no licensed vaccine or antiviral drug for DF (13). This virus can infect anyone, but it tends to be more severe in immunocompromised individuals (14). Although exposure to the DENV provides lifelong immunity against the specific virus serotype to which the patient was exposed, in countries with periodic dengue epidemics, these epidemics are geographically distributed, creating different serotypes in the population (5, 14).
In other words, the circulation of DENV serotypes among patients and genetic recombination, along with the high mutation rate of the DENV genome, increases the likelihood of genetic changes. The diversity of virus populations may lead to the emergence of new DENV serotypes (2). Although the clinical criteria for DF are known, DF remains a cryptic disease that can present with a wide variety of clinical signs and manifestations related to virus-vector and host-virus interactions (14).
Individuals with dengue infection can exhibit a range of clinical symptoms, from mild fever (DF) to severe hemorrhagic diseases such as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) (5). Meanwhile, DSS and DHF are more serious and severe forms of the disease, associated with hemostatic disorders and increased vascular permeability, which can cause bleeding in the body. This condition is life-threatening and may progress to its most critical form, dengue hypovolemic shock syndrome, a potentially fatal disease (5, 15).
Clinical symptoms of DF include high fever, headache, body aches, muscle pain, joint pain, vomiting, temporary rashes, petechiae, ecchymosis under pressure, and bleeding of blood vessels. In more severe stages, plasma leakage may lead to shock and fluid accumulation with severe bleeding. For DF, antipyretics and painkillers can be used to relieve pain and discomfort in the skeletal system, and in more severe cases, hospitalization and fluid therapy are required (5).
2. Objectives
In this study, we aimed to present a summary of the clinical symptoms, pathology, etiology, prevention, and treatment options based on previous studies, to facilitate a better understanding of DF.
3. Methods
In the present study, research articles related to DHF from 1989 to 2024 were searched and analyzed. Specialized keywords such as Dengue, DENV, DENV infection, DF infection, DHF, dengue vector, etiology, diagnosis, prevention and treatment of DF, human dengue infection, pathologic study of dengue, pathogenesis of DENV infection, epidemiology of DF, DF control, dengue vaccines, renal injury in DF, respiratory sequelae of DF, DENV-induced liver injury, and encephalopathy syndrome associated with DF were used for searching. These were analyzed in Google Scholar, PubMed, and other specialized databases.
The criteria for including research articles were that they were published in English in reputable domestic or international scientific journals and were related to DF.
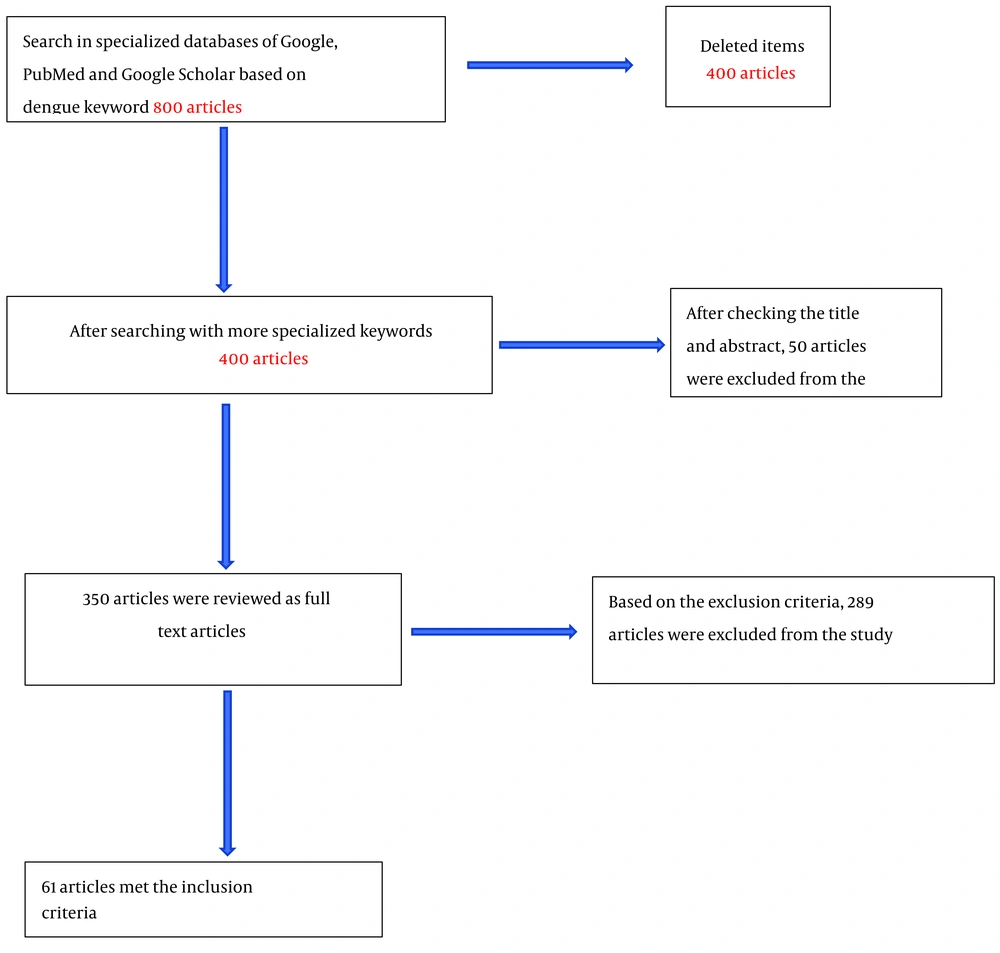
The criteria for excluding articles were those published in non-reliable journals or those whose statistical population was not focused on DF. Approximately 800 articles were found in the initial search using the general keyword "dengue." After refining the search with specialized keywords, about 400 articles were selected for the next stage. After reviewing the titles of these articles, 350 were chosen for full-text review. Finally, after reading the full texts and applying the exclusion criteria, 61 articles that met the desired characteristics were considered the final articles for our study (Figure 1).
The PEDro scale was used to assess the quality of the articles. This scale consists of 11 questions, with one point awarded for each question. If an article receives a score of 7 or above, it indicates a high level of quality. A score between 5 and 6 indicates an average quality, and a score below 5 indicates a weak quality (16).
4. Epidemiology and Etiology
Men are more affected by DF than women. Additionally, the middle-aged group accounts for the majority of patients (17). Currently, over 100 tropical and subtropical countries are endemic to DF, and more than a third of the world’s population is at risk of infection with this febrile and dangerous virus (18). As a result, DF has become a significant global concern. However, the key drivers of the dengue epidemic remain unclear (19).
Climate change (global warming) is expected to increase the Earth's temperature by 2.5 - 2.9 degrees Celsius by the end of the century, which will intensify disease transmission (20).
Humans are the primary host of the virus. After a female mosquito bites and feeds on a person infected with DENV, the virus multiplies in the mosquito's midgut and enters the salivary glands. When the virus is transferred to healthy individuals through the mosquito bite, it infects various immune cells, such as macrophages, dendritic cells, and Langerhans cells. These infected cells migrate to the lymph nodes, where they interact with new cells, causing further infections that lead to viremia (21, 22). A single infected mosquito bite is enough to spread the infection. Exposure to infected blood, organs, or other tissues can result in blood-borne transmission of the virus, producing a 7-day viremia in humans (e.g., bone marrow). Congenital transmission of DF has not been reported. A baby can become infected during birth through microtransfusion after the separation of the placenta or mucosal contact with the mother’s contaminated blood. Breast milk may also be a potential source of DENV transmission to babies. There is no evidence to suggest that the virus can be transmitted through sexual contact (23).
Most of the effects of DF impact major organs such as the liver, lungs, kidneys, heart, and brain. Although neurologic manifestations of DF are rare, they can include intracranial hemorrhage, encephalitis, and acute disseminated encephalomyelitis (ADEM) (24). While DF typically occurs on its own, it can also manifest simultaneously with rickettsial infection, malaria, chikungunya, and COVID-19 in patients. Co-infection leads to overlapping signs and symptoms, which can make it difficult for doctors to diagnose and treat the disease properly. A delayed diagnosis of co-infection may result in serious complications for the patient (25). Dengue fever is generally characterized by a self-limiting fever lasting 5 to 7 days, which can be debilitating for the patient during the acute stage. Liver involvement is more common in men and older adults and is often associated with DEN serotype 2 and secondary infections.
5. Clinical Symptoms
According to the classification by the World Health Organization (WHO) in 1997, DF is classified into DF, DHF, and DSS (Table 1).
| Row | Classification | Clinical and Laboratory Signs |
|---|---|---|
| 1 | DF | Eye pain, headache, muscle or joint pain, skin rash, bleeding manifestations and decreased leukocyte count |
| 2 | DHF | Fever, thrombocytopenia (≤ 100 × 109/L), bleeding manifestations, and evidence of plasma leakage |
| 3 | DSS | Tachycardia or low pulse pressure (< 20 mm Hg) or hypotension (systolic blood pressure < 90 mm Hg) |
World Health Organization Classification in 1997
In 2009, the revised WHO classification included dengue with or without warning signs, as well as severe dengue (Table 2) (21).
| Row | Classification | Clinical and Laboratory Signs |
|---|---|---|
| 1 | Dengue without warning signs | Fever, nausea, vomiting, skin rash, body ache, leukopenia |
| 2 | Dengue with warning signs | Abdominal pain or the presence of tenderness, persistent vomiting, clinical evidence of fluid accumulation like pleural effusions and ascites, bleeding, lassitude or restiveness, hepatomegaly, or rise in packed cell volume (PCV) (≥ 20%) with a rapid reduction in thrombocyte count (< 50 000/mm3) |
| 3 | Severe dengue | Severe plasma leakage, bleeding and disorders in vital organs such as liver involvement in the form of an increase in transaminases over 1000 IU/L and central nervous system manifestations such as changes in sensory or cardiac involvement or other organs. |
Revised World Health Organization Classification in 2009
Clinical symptoms vary depending on the age of the patient. In infants and young children, the symptoms can differ (Table 3) (26).
| Row | Age and Gender of the Patient | Clinical and Laboratory Signs |
|---|---|---|
| 1 | Infants and young children | Undifferentiated fever and maculopapular rash, Leukopenia and thrombocytopenia |
| 2 | Older children and adults | High fever (biphasic), severe headache, discomfort behind the eyes, myalgia, arthralgia, nausea, vomiting. In some patients, dengue fever is associated with bleeding complications such as epistaxis, bleeding gums, bleeding in the gastrointestinal tract, hematuria, Leukopenia and thrombocytopenia. |
| 3 | Women | In addition to the clinical and laboratory symptoms mentioned in row 2, menorrhagia is also seen in women. |
Clinical Symptoms Depending on the Age of the Patients
Eighty percent of people infected with the DENV are asymptomatic or experience mild symptoms, such as a mild fever. The incubation period is usually between four and seven days; however, it can last up to fourteen days. Symptoms of DF include high fever (40°C), severe headache, eye pain, muscle and joint pain, nausea, vomiting, swollen glands, and rashes (27).
In cases of liver involvement with DF, clinical features such as abdominal pain, hepatomegaly, jaundice, nausea/vomiting, and echogenic liver (showing necrosis of liver cells and minimal inflammation) are observed (28). Common lab findings include increased levels of aspartate aminotransferase and alanine aminotransferase, elevated bilirubin, alkaline phosphatase, gamma-glutamyl transferase, creatinine, and creatine kinase, as well as decreased long-term albumin, prothrombin, activated partial thromboplastin time, platelet count, metabolic acidosis, and an increased hematocrit in dengue liver patients (21, 27, 28).
According to the WHO, the most common neurological complication resulting from systemic dengue infection is encephalopathy. Individuals with dengue encephalopathy are diagnosed with changes in consciousness, seizures, mental confusion, neck stiffness, limb spasticity, positive clonus, hemiplegia, respiratory distress, shock, and a positive Kernig's sign (26).
Although cardiac involvement by DENV is considered a rare complication, the most concerning cardiac disorder is dengue myocarditis (29). Clinical symptoms of dengue myocarditis include bradycardia, shortness of breath, chest pain, atrial block, T wave abnormalities, ST segment changes, and sudden death (30, 31). The main pathophysiological factors associated with myocardial dysfunction include edema caused by capillary leakage, circulating myocardial suppressive factors (such as inflammatory mediators), coronary hypoperfusion, and altered calcium homeostasis (29).
Renal disorders in patients with DF range from serum electrolyte abnormalities to hematuria and proteinuria, as well as acute kidney injury (AKI) and hemolytic uremic syndrome, depending on the severity of the disease (32).
Clinical symptoms of dengue-related pulmonary involvement include pleural effusion, non-cardiogenic pulmonary edema, pneumonitis, acute respiratory distress syndrome, and pulmonary hemorrhage (33).
6. Pathology and Pathophysiology
The E protein is involved in virus binding (21). After the DENV enters the body, it first infects Kupffer cells. The infected Kupffer cells undergo apoptosis through the production of nitric oxide and interferon-alpha, and a few hours later, through pathways mediated by interleukin-6 and tumor necrosis factor-alpha (34). The viral replication rate is higher in HepG2 liver cells, which is why children are more susceptible to severe DF, as most of their liver cells are in the G2 phase (21).
In vitro studies have shown that apoptosis induced by hepatocyte infection with the DENV occurs through activation of the p38-dependent NF-κB transcription factor, which is induced by endoplasmic reticulum stress. The homologous protein with CCAAT binding protein acts as a transcription factor enhancer (35).
The pathological features of DHF and DSS include perivascular edema without destruction of the vascular endothelium, lobular necrosis of the liver, necrosis of the spleen parenchyma and lymph nodes, and hyperplasia of thymus cells (15). Other findings include mild to moderate hyperplasia and hypertrophy of Kupffer cells, monocyte infiltration of portal tracts, and infiltration of inflammatory cells (lymphocytes, plasma cells, neutrophils, and macrophages), with a higher frequency of lymphoplasmic infiltration, especially around the foci of necrosis. Additional features include macrovesicular steatosis, hepatocytic ballooning, and lytic necrosis (36). The clinical features of DHF include plasma leakage, a tendency to bleed, liver involvement, pleural or other effusions, hypoalbuminemia, and hypoproteinemia. Liver tissue may be enlarged, and serum aminotransferase activity is usually elevated (15). Among patients with arboviral infections, changes in the portal vein ratios in DF, yellow fever (YF), and chikungunya fever (CF) are high (30).
In fatal DENV infection, DENV antigens can be detected in the cytoplasm of phagocytic mononuclear cells in various tissues, including the liver, spleen, brain, and lungs (15). In cases of death from this virus, liver cells in the necrotic areas show positive DENV antigen (37, 38). The DENV antigens can be detected in both Kupffer cells and liver cells (39). Liver damage caused by DF becomes more noticeable as the disease progresses to a more severe form (21).
Dendritic cells (DCs) increase the production of pro-inflammatory cytokines and stimulatory molecules that play a crucial role as mediators between innate and adaptive immune responses during viral invasion, thus stimulating immune responses in humans. The DENV genome encodes seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) and three structural proteins (C, E, and prM/M) after translation into a single polyprotein. The replication process of the viral genome in the host cell is primarily driven by the NS proteins. NS1, which is a strong antagonist of the host's antiviral interferon responses, can limit the migration and maturation of dendritic cells. By regulating the expression of the genome, it prevents the induction of IFN-γ release from Th1 cells during the immune response, which are key players in controlling the initial phase of DENV viral replication (26, 40, 41). The pathophysiology of liver tissue involvement in DF is still not fully understood (21).
Overproduction of cytokines, including IL-1β, TNF-α, IL-6, IL-8, and IL-10, along with enzymes such as matrix metallopeptidase (MMP2) and chemotactic proteins like IP2 and RANTES during DENV infection, leads to the formation of complexes between DENV and antibodies. This, in turn, activates the complement system by increasing the levels of anaphylatoxins C3a and C5a. Direct infection of endothelial cells causes dysfunction and damage to these cells, increasing vascular permeability and fluid leakage. This contributes to cerebral edema in the central nervous system (CNS) (26, 42-44). Additionally, antibodies against protein E and NS1 can identify and attack plasminogen and various coagulation proteins, as well as endothelial cells, disrupting and destroying the barrier that regulates vascular permeability and endothelial cell adhesion (45-47). The production and secretion of IL-1β by platelets during DENV infection leads to the release of nitric oxide (NO), a potent vasodilator that increases endothelial permeability (29).
The accumulation of immune cells, severe acute congestion, and increased vascular permeability can be observed in the samples from all individuals infected with the DENV (48).
7. Diagnosis
In endemic areas, dengue screening is often conducted clinically based on clinical symptoms and physical examination, as well as using ultrasound for the early diagnosis of DSS resulting from pleural effusion or ascites in patients (48). However, distinguishing primary dengue from other viral infections may be challenging (34). Fever with nausea and vomiting, along with symptoms such as skin rash, low white blood cell count, widespread pain, positive tourniquet test, or any warning signs in individuals from endemic areas, may indicate a possible diagnosis (27).
Today, diagnostic techniques such as laboratory tests and imaging are used to diagnose DF, as summarized in Table 4.
| Row | Diagnostic Techniques | Description |
|---|---|---|
| 1 | Laboratory tests | PCR, RT-PCR, ELISA, IC, IH |
| 2 | Imaging studies | MRI, CXR, CT |
Dengue Fever Diagnostic Techniques
Further investigation of hematological parameters is needed for better diagnosis and treatment of DF (17). Disease diagnosis is often made through virus isolation in cell cultures, nucleic acid (viral RNA) detection using PCR, viral antigen detection (such as using NS1), or specific antibodies (27, 34). However, the sensitivity of NS1 antigen detection during the febrile phase of primary infection may be higher than 90%, while in secondary infection, it is only 60 - 80% (49). Detection of the NS1 antigen in blood samples using enzyme-linked immunosorbent assay (ELISA) and rapid immunochromatography (IC) are simple and inexpensive tests that can be used in clinical settings to diagnose DF (for primary infection until the ninth day and secondary infection until the seventh day) (29, 50). After the fifth day of the disease, hemagglutination inhibition (HI) and ELISA tests can be used to detect dengue-specific IgM and IgG antibodies (29). In primary infections, IgM can be detected in serum samples from the fourth day, with levels increasing until the sixth day, remaining positive for several months. After 14 to 21 days, the level of IgG in the blood increases. The specific IgG titer, in the absence of symptoms, is considered a reliable marker for the diagnosis of previous dengue infection (more than 60 days). The presence of specific IgM in a patient with clinical symptoms is considered diagnostic (27, 29, 51, 52). In secondary infection, the IgG titer increases rapidly from day 3, and the IgM level may be undetectable (29). According to WHO recommendations, DF should not be diagnosed until a blood sample is taken at a 14-day interval, showing evidence of serum conversion from IgM to IgG, and a more than four-fold increase in specific IgG levels; IgG alone cannot be considered diagnostic (27, 29).
In individuals with DENV, the CNS can be affected, and tests such as anti-DENV immunoglobulin (Ig) M or NS1 can be detected in the cerebrospinal fluid (CSF). These tests can isolate the virus from the CNS and distinguish it from other agents that cause viral brain diseases (26).
Dengue brain involvement can be diagnosed through PCR, reverse transcription polymerase chain reaction (RT-PCR) (29), and immunological tests in serum/CSF (identification of dengue NS1 antigen, DENV, and DENV-specific IgM antibodies in CSF). Additionally, brain MRI can be used to diagnose bleeding caused by dengue encephalitis and to examine various brain regions, including the basal ganglia, hippocampus, temporal lobes, cerebellum, thalamus, brain white matter, brainstem (especially the substantia nigra), and cerebellum. The CSF lymphocytic pleocytosis is found in 85% of patients (53). It should be noted that due to the decrease in CSF virus concentration, the PCR method may yield average results. On the other hand, the sensitivity of immunological tests in CSF is limited (26, 53). Therefore, the diagnosis of dengue encephalitis is typically based on clinical suspicion of dengue, confirmation of systemic DENV infection, symptoms of encephalitic syndrome (with or without abnormal CSF findings), and abnormal brain MRI (26).
In cases of dengue lung injury, chest x-ray (CXR) can be used to diagnose pleural effusion and consolidation, while ultrasound or computed tomography (CT) is beneficial for diagnosing pleural effusion and consolidation (54). Additionally, ELISA and the presence of NS1 antigen, IgG, and IgM specific to the DENV, reverse transcription polymerase chain reaction, and hemagglutination inhibition can be used for rapid laboratory diagnosis (55). However, the limitations of current diagnostic methods include their unavailability in many medical diagnostic settings and the high cost of molecular and serological diagnostic tests.
8. Prevention and Treatment
Eradication of Aedes aegypti habitats by removing open water sources or using insecticides and biological control agents is the primary strategy for preventing dengue disease (27, 56). Additionally, wearing clothing that covers the skin completely, sleeping under a mosquito net, and/or using insect repellent are effective methods for preventing mosquito bites (56).
In most cases, DF is often misdiagnosed or overlooked due to its similarity to other febrile diseases, such as malaria (27). The discovery of DENV-5 and the potential emergence of new serotypes in the future, due to genetic recombination, natural selection, and genetic bottlenecks, may hinder progress in the dengue vaccine initiative (2).
Dengvaxia is the only approved DENV vaccine. Both Dengvaxia and TAK003 (DENVax) are the only DENV vaccines that have been used in children, although they have shown mixed results. Therefore, early diagnosis, preventive measures, and supportive care are essential in managing DF and minimizing its impact on public health (57). So far, the Dengvaxia vaccine has been approved and licensed in several countries. This vaccine is a weak combination of the four dengue serotypes and the yellow fever virus (58). It is only effective in individuals with a history of dengue infection (27).
Although no specific antiviral drug exists for DF, the WHO recommends fluid therapy with crystalloid solutions for intravascular fluid volume balance in cases of dengue shock (10 mL/kg for every 1% of body weight loss), as long-term shock and fluid overload are associated with high mortality rates (20). Patients with severe dengue should be treated in a facility with access to an intensive care unit (ICU) (27). In children with dengue shock, a rapid dose of 20 mL/kg is appropriate (59). The rate of fluid delivery is adjusted after the stabilization of vital signs, hematocrit, and urinary output, which should be maintained at 0.5 - 1 mL/kg/h (60).
Fluid replacement should be carefully monitored, and platelet transfusions are required in cases of severe bleeding (26). There is no specific treatment for patients with dengue encephalitis or encephalopathy. The treatment of dengue encephalitis focuses on managing the viral infection and inflammation, while the treatment of dengue encephalopathy focuses on correcting systemic metabolic disorders (61).
An in vivo study showed that metformin reduced the risk of DF in diabetic patients using metformin compared to non-users. Therefore, metformin may be a promising antidengue agent (36).
In cases of renal failure in patients with dengue, the administration of fluids should be carefully monitored (33). In some cases of renal involvement due to DF, hemodialysis may be required to restore fluid balance (32).
9. Conclusions
Dengue is a dangerous disease for public health, particularly in tropical and subtropical regions, and a definitive solution for its prevention and treatment has not yet been discovered. Given the importance of this issue, the research conducted, and the potential future directions for understanding dengue, prevention and treatment should be prioritized. It is necessary to increase awareness among the populations in affected regions through social media. Furthermore, government agencies should focus on eliminating breeding sites of Aedes aegypti and Aedes albopictus, as reducing these habitats will help lower the risk of disease transmission. Future studies and joint efforts between governments and health organizations are essential in addressing the challenges posed by DF.