1. Background
Nowadays, cancer is one of the most significant diseases and has a high mortality rate. Colon cancer is a common and fatal disease that can be prevented (1). It ranks as the fifth most common cancer globally, with nearly 1.2 million new cases and 0.6 million related deaths reported in 2020 (2). In 2018, the World Health Organization (WHO) reported that 1.80 million new cases of colorectal cancer were diagnosed worldwide, resulting in 862,000 deaths (3). Colorectal cancer is currently the third most common cancer diagnosis among both men and women in the United States (4). Approximately 50,000 patients die from colorectal cancer annually in the United States (5).
The prevalence of colon cancer is increasing in Iran, where it is considered one of the most important cancers affecting both sexes (6). About 30% to 40% of cases occur in individuals under the age of 50 years, and patients with colorectal cancer are more likely to have a family history of various cancers (7).
The risk of developing colorectal cancer may increase due to environmental and genetic factors. Various risk factors influence the development of colorectal cancer, including diabetes, obesity, inactivity, a low-fiber and high-fat diet, smoking, alcohol consumption, age over 50, low socioeconomic status, personal or family history of colorectal cancer, long-term inflammatory bowel disease (IBD), familial adenomatous polyposis (FAP), and mutated MMR gene syndromes (8, 9). Additionally, the main factors that contribute to cancer progression, recurrence, and metastasis are colon cancer stem cells (CCSCs). The accumulation of genetic and epigenetic changes, along with communication with the tumor microenvironment (TME), can lead to the evolution of these cells into fully malignant cells (10, 11).
Currently, colon cancer is one of the few completely preventable cancers. According to studies from the University of California, an 80% increase in colonoscopy rates from 1997 to 2002 led to a decrease in the incidence of colon cancer from 42 per hundred thousand to 38 per hundred thousand; however, less than half of the eligible U.S. population is routinely screened (12, 13). Colorectal cancer screening and early detection, along with the removal of colon adenomatous polyps and access to more appropriate treatments, have contributed to a decrease in both incidence and mortality (14).
The enteric nervous system consists of the myenteric (Auerbach's) plexus and submucosal (Meissner's) plexus. The myenteric plexus is located between the longitudinal and circular muscle layers and facilitates gastrointestinal tract movement. In contrast, the submucosal plexus is responsible for secretion and innervates intestinal endocrine cells, glandular epithelium, and submucosal blood vessels beneath the mucosal layer. One possibility for the varying prevalence of nerve invasion among different cancer types is the normal innervation of each organ from which the tumor originates (15, 16). A study by Huh et al. demonstrated that the positivity of both lymphovascular invasion and perineural invasion (PIN) is a strong predictor of overall and disease-free survival in patients with stage II and III colorectal cancer (17).
2. Objectives
Given the insufficient information regarding PIN and its significance in colorectal and other types of cancer, this study was conducted to investigate the relationship between PIN and tumor stage in patients with colorectal cancer.
3. Methods
In this cross-sectional study, all patients with colorectal adenocarcinoma who were sent to the Pathology Department of Shahid Sadoughi Hospital between 2014 and 2022 were included. The inclusion criteria comprised confirmation of colorectal adenocarcinoma by a pathologist, inclusion in the patient file, and completeness of the demographic information in the file. The exclusion criteria included any incompleteness of the main information in the file. The total sample size consisted of 169 patients.
Based on the entry and exit criteria, all patient records were reviewed, and the required information was extracted from these records. The database included a comprehensive array of variables, such as mortality status, tumor anatomical site, grade and stage, recurrence status, survival rate, and PNI.
The collected data were analyzed using SPSS statistical software version 22. The χ² test was employed to compare the frequencies and proportions of categorical factors to determine clinicopathological characteristics. Logistic regression analysis was conducted to predict PNI by applying the measured variables. A significance level of less than 0.05 was considered statistically significant.
4. Results
The age of the patients was 58.8 ± 17.8 years, with a range from 19 to 93 years. Among the patients, 89 (52.7%) were male and 80 (47.3%) were female. Most patients (81, 55.5%) were alive, while 65 (44.5%) had died. According to the findings, 8.3% (14 patients) had nerve invasion, whereas 91.7% (155 patients) had no nerve invasion. The most common anatomical location for colon cancer was the sigmoid colon (50 patients; 29.6%). The clinicopathological information of the patients according to the PNI categorization is reported in Table 1. Most negative recurrence cases exhibited no PNI, and a significant relationship was observed between stage classification and PNI (P < 0.05). Additionally, the survival time among women was slightly higher than that among men (1.84 vs. 1.81), although this difference was not statistically significant (P = 0.923).
| Variables | PNI; No. (%) | P-Value | |
|---|---|---|---|
| Negative | Positive | ||
| Age | 0.047 a | ||
| 19 - 39 | 25 (16.1) | 2 (14.3) | |
| 40 - 59 | 50 (32.3) | 9 (64.3) | |
| 60 - 79 | 58 (37.4) | 3 (21.4) | |
| 80 > | 22 (14.2) | - | |
| Sex | 0.578 | ||
| Male | 83 (93.3) | 6 (6.7) | |
| Female | 72 (90) | 8 (10) | |
| Tumor size | 0.904 | ||
| < 1 | 37 (24) | 4 (28.6) | |
| 1.1 - 2 | 9 (5.8) | 1 (7.1) | |
| 2.1 > | 108 (70.1) | 9 (64.3) | |
| Recurrent | 0.413 | ||
| No | 88 (89.8) | 10 (10.2) | |
| Yes | 58 (93.5) | 4 (6.5) | |
| Recurrent location | 0.29 | ||
| Colon | 37 (90.2) | 4 (9.8) | |
| Non colon | 21 (100) | - | |
| Grade | 0.136 | ||
| Mild | 68 (95.8) | 3 (4.2) | |
| Moderate | 65 (86.7) | 10 (13.3) | |
| Severe | 13 (92.9) | 1 (7.1) | |
| Stage | 0.014 a | ||
| I | 37 (100) | - | |
| II A | 44 (97.8) | 1 (2.2) | |
| II B | 2 (100) | - | |
| III A | 2 (100) | - | |
| III B | 26 (81.3) | 6 (18.8) | |
| III C | 5 (71.4) | 2 (28.6) | |
| IV A | 15 (83.3) | 3 (16.7) | |
| IV B | 16 (88.9) | 2 (11.1) | |
Comparison of Perineural Invasion According to Clinicopathological Features (N = 169)
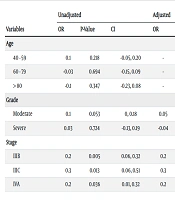
In the univariate logistic regression model for predicting PNI, the moderate grade and tumor stages IIIB, IIIC, and IVA showed significant relationships, whereas age was not significantly related to PNI. Consequently, in the multivariate analysis, tumor stage and grade were evaluated. No significant differences were observed in grade classification within the multivariate model. The likelihood of PNI was decreased in stage IIIC compared to the others (OR adjusted for significant relation = 0.3, CI 95%: 0.03, 0.49) (Table 2).
| Variables | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| OR | P-Value | CI | OR | P-Value | CI | |
| Age | ||||||
| 40 - 59 | 0.1 | 0.218 | -0.05, 0.20 | - | - | - |
| 60 - 79 | -0.03 | 0.694 | -0.15, 0.09 | - | - | - |
| > 80 | -0.1 | 0.347 | -0.23, 0.08 | - | - | - |
| Grade | ||||||
| Moderate | 0.1 | 0.053 | 0, 0.18 | 0.05 | 0.34 | -0.05, 0.14 |
| Severe | 0.03 | 0.724 | -0.13, 0.19 | -0.04 | 0.68 | -0.2, 0.13 |
| Stage | ||||||
| IIIB | 0.2 | 0.005 | 0.06, 0.32 | 0.2 | 0.017 | 0.03, 0.31 |
| IIIC | 0.3 | 0.013 | 0.06, 0.51 | 0.3 | 0.025 | 0.03, 0.49 |
| IVA | 0.2 | 0.036 | 0.01, 0.32 | 0.2 | 0.051 | -0.01, 0.32 |
Logistic Regression Model of Cliniopathological Variables to Predict Perineural Invasion Tumor
5. Discussion
The study's findings indicate that the disease has a low onset age and that the patients are young; the patients in this study had an average age of 58.8 ± 17.8 years. This is considerably lower than that in Western countries and is close to the average age of Chinese patients (18). In 2007, a report from America examined racial differences in 13,758 cases of colorectal cancer; the rate of colorectal cancer before the age of 50 was 7% in whites, 12.5% in blacks, and 17.1% in immigrants from Asian Pacific Islands (19). In Western societies, only 6.8% of people with colorectal cancer are under 40 years of age, and 20% are under 50 years of age (20). The onset of the disease in our society seems to be accelerated by environmental factors, such as certain eating habits (increasing the consumption of carbohydrates and fats while reducing the consumption of fiber), especially in young people, changes in lifestyles involving insufficient movement and obesity, the youth of the majority of the population, and the involvement of genetic issues. Regular physical activity has been shown in many studies to have a preventive effect against colorectal cancer, whereas obesity increases the risk. Evidence suggests that a diet high in fruits and vegetables lowers the chance of developing PNI colorectal cancer, while a high intake of fat, red meat, alcohol, and smoking increases the risk of developing PNI (21).
In this study, a significant relationship between age and perineural nerve invasion was observed, with the most common age group exhibiting nerve invasion being between 40 and 59 years old. According to this study, the ascending and sigmoid colon are the most common anatomical locations for colorectal cancer. Moreover, available sources in this field indicate that women are more susceptible to tumors on the right side of the colon, while rectal cancer is more prevalent in men (22).
In our study, the mean survival time of patients after a diagnosis of colorectal cancer was 1.82 years. Based on the results of a study conducted in Iran in 2008, the average survival time of patients after a diagnosis of colorectal cancer was reported to be 3.5 years. The survival rate of patients with colorectal cancer in Iran is roughly 41% of that of patients with this type of cancer in developed countries (23). Similar to our study, a systematic review and meta-analysis study in Iran indicated that women had a better survival rate than men. This may be related to the higher participation of women in screening programs such as fecal occult blood tests and colonoscopy (24-26). In addition, gonadal hormones and testosterone, as protective factors, can influence the immune system and immunological response (27).
PNI can be easily identified and diagnosed under a microscope with hematoxylin and eosin staining, demonstrating good reproducibility. Perineural invasion can be detected in 10 - 35% of tumor samples resected from colorectal cancer (CRC), as previously reported, and it increases with higher tumor grade and stage. In the present study, the rate of positive PNI diagnosis was 8.3%. PNI reflects the aggressive nature of the tumor, and the classification of patients based on TNM staging, along with their PNI status, will be very useful for determining adjuvant clinical treatment (28). The results of univariate logistic regression analysis indicated that stage and grade were independent risk factors for PNI in patients diagnosed with colorectal cancer (CRC). Specifically, stage IIIC can decrease the odds of PNI by 30% compared to other stages and is considered a protective factor. A study in Changde supports our findings, showing that tumor histological differentiation (grade) could be a risk factor for PNI (29).
Although TNM staging for CRC has been widely utilized in evaluating patient prognosis, some issues still need to be addressed. For example, patients with the same TNM stage may have different prognoses, especially for stage II and III patients, where this is often the case. The neurological invasion of CRC has attracted more research attention, but no consensus has yet been reached on specific details (30). Liebig et al. reported that patients with stage II PNI had a poorer prognosis than those with stage III (31). Some studies have shown that the survival rate of stage II CRC patients with PNI (+) is not only significantly worse than that of stage II CRC patients with PNI (-), but also worse than that of stage III patients. Furthermore, some authors have reported that stage II CRC patients with PNI (+) and stage III CRC had similar survival rates (21, 32, 33). In this study, the relationship between PNI and tumor stage was statistically significant, and most nerve invasions were observed in stages IIB, IIC, IVA, and IVB. The results of this study showed no significant difference in nerve invasion between males and females, similar to internal studies (21). However, in the Vakili study in Yazd, it was found to be more common in males (21). Tumor progression, including local or distant recurrence, is the most common cause of death in patients with CRC. The mechanism behind the association between PNI and tumor progression is not well understood (34). The results from Chu and Leijssen are inconsistent with ours; their findings indicated that male sex, tumor site, and tumor grade were correlated with PNI. This discrepancy may be due to differences in study type and sample size (35, 36).
5.1. Conclusions
In this study, no statistically significant relationship was observed between perineural invasion and recurrence or the site of recurrence. However, a significant relationship was observed between perineural invasion and both age and tumor stage. In the regression model, grade and tumor stage had a relationship with PNI, and these factors could predict the likelihood of PNI. In summary, this study further demonstrated that PNI can be considered an independent poor prognostic factor for colorectal cancer (CRC). It is suggested that similar studies be conducted with larger sample sizes in different locations.