1. Introduction
Acute pancreatitis (AP) during pregnancy is a rare condition, with an estimated incidence of one case per 1,000 to 5,000 pregnancies (1). It is more common in multiparous women, typically occurring in the third trimester or early postpartum period (50% and 38%, respectively) (2). The causes of AP in pregnancy are similar to those in non-pregnant individuals and include gallstones (65 - 100%), alcohol abuse (5 - 10%), idiopathic AP (15%), and hypertriglyceridemia (HTG)-induced pancreatitis (5%) (3). Less common etiological factors include hyperparathyroidism, infections, certain medications, and injuries.
Acute pancreatitis during pregnancy has historically been associated with high maternal and fetal mortality rates (37% and 60%, respectively), though these rates have decreased significantly to 3% for maternal mortality and 11.6% - 18.7% for fetal mortality, due to earlier diagnosis and advances in maternal and fetal intensive care (1, 4). Development of AP in the first trimester is linked to higher maternal and fetal mortality rates. The reported maternal mortality rate was 12.7% in the first trimester, compared to 7.9% and 6.4% in the second and third trimesters, respectively (4). First-trimester onset also correlates with the lowest rate of term pregnancies (60%) and the highest risks of fetal loss (20%) and preterm delivery (16%) (5).
Hypertriglyceridemia-induced pancreatitis accounts for 1 - 7% of AP cases in pregnancy and is responsible for 56% of gestational pancreatitis cases (6). The most common risk factors for HTG in pregnancy are pre-existing dyslipidemia, hypertension, diabetes, and alcohol use, although some cases of HTG in pregnancy occur without any identifiable predisposing factors (1). Here, we report a case of early-onset HTG-induced pancreatitis in a pregnant woman without any known predisposing factors for HTG.
2. Case Presentation
A 29-year-old primigravid woman presented at 21 weeks of gestation to the obstetrics emergency department with a complaint of acute, severe, persistent epigastric and left upper abdominal pain that had started one day prior. The pain was positional, radiating to her back, worsened by lying supine or eating, and was accompanied by nausea and recurrent vomiting.
Upon presentation, the patient was conscious but appeared ill, with tachycardia (pulse: 124/min) and mildly elevated blood pressure (systolic BP, 135 mmHg; diastolic BP, 85 mmHg). Her temperature and respiratory rate were normal (37.1°C and 18/min, respectively). On physical examination, tenderness was noted in the epigastric area and left upper quadrant. Ultrasonography revealed a single fetus with normal amniotic fluid (amniotic fluid index, 16 cm) and a fetal heart rate of 148 beats/min. Aside from a three-year history of primary infertility, she had no significant medical or surgical history, and her family history was unremarkable for any specific disease. Her pregnancy was achieved via in vitro fertilization, and she had only been taking folic acid supplements during pregnancy.
The differential diagnoses included acute pancreatitis, gallstones, peptic ulcer disease and its complications, diabetic ketoacidosis, and preeclampsia.
Initial laboratory examinations revealed leukocytosis with a left shift, mild hypernatremia, elevated serum amylase levels, severe HTG, ketonuria, proteinuria, normal liver and renal function tests, and normal random blood glucose levels. Severe lipemia prevented an initial hemoglobin measurement. Venous blood gas analysis indicated a mixed metabolic acidosis and respiratory alkalosis (Table 1). Abdominal ultrasonography showed an enlarged pancreas with homogenous parenchyma.
| Lab. Parameters | First Day of Add | 2nd Trimester Specifics Normal Range |
|---|---|---|
| WBC | 17400 | 5.6 - 14.8 (× 103/mm3) |
| Hb | Not available due to lipemia | 9.7 - 14.8 (g/dL) |
| Hct | 32.1 | 30- 39 (%) |
| Plt | 304 | 155 - 409 (× 109/L) |
| ESR | 31 | 7 - 47 (mm/hr) |
| CRP | 0 | 0.4 - 20.3 (mg/L) |
| Amylase | 1170 | 16 - 73 (U/L) |
| BUN | 12 | 3 - 13 (mg/dL) |
| Cr | 0.7 | 0.4 - 0.8 (mg/dL) |
| PTT | 36 | 22.9 - 38.1 (Sec) |
| PT | 13 | 9.5 - 13.4(Sec) |
| INR | 1 | 0.83 - 1.02 |
| AST | 50 | 3 - 33 (U/L) |
| ALT | 15 | 2 - 33 (U/L) |
| ALP | 180 | 25 - 126 (U/L) |
| Bilirubin Total | 1.3 | 0.1 - 0.8 (mg/dL) |
| Bilirubin Conjugated | 0.45 | 0 - 0.1 (mg/dL) |
| LDH | 500 | 8 - 447 (mg/dL) |
| TG | 8100 | 75 - 382(mg/dL) |
| CHOL | 140 | 176 - 299 (mg/dL) |
| PH | 7.38 | 7.4 - 7.52 |
| HCO3 | 13.1 | Not reported |
| Pco2 | 22 | Not reported |
| NA | 148 | 129 - 148 (mEq/L) |
| K | 5 | 3.3 - 5 (mEq/L) |
Based on the patient’s clinical presentation and paraclinical findings, the diagnosis of HTG-induced pancreatitis was confirmed. She was admitted to the intensive care unit, where a multidisciplinary team comprising a gastroenterologist, an endocrinologist, and an obstetrician managed her treatment.
The therapeutic protocol was initiated as follows:
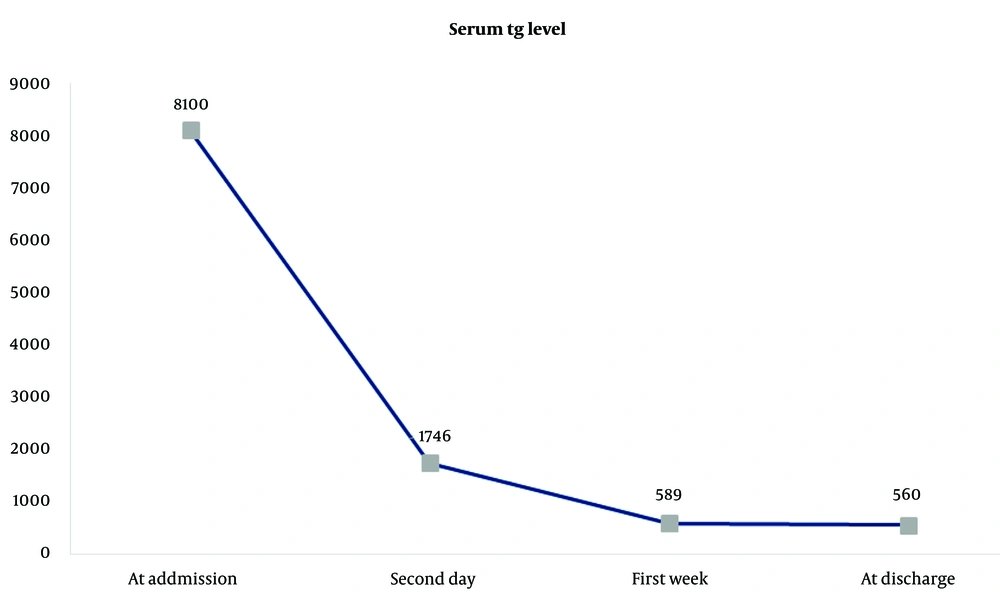
The patient was kept fasting with correction of fluid and electrolyte imbalances and administered analgesics. To reduce serum triglyceride levels, a combination of low molecular weight heparin (enoxaparin 6000 units/day) and an insulin/potassium/dextrose infusion (infusion rate: 2 - 6 units/hr of regular insulin, 10 cc of KCL 15% in each liter of IV fluid, and 150 - 300 cc/hr of D5W) was immediately started and continued for 11 days until discharge. As an initial therapeutic measure, two sessions of plasma exchange were performed to expedite the reduction in serum triglyceride levels. Each plasma exchange session involved 2.5 liters of exchange fluid containing 1250 cc of normal saline with 4 vials of 25% albumin and 1250 cc of fresh frozen plasma (FFP). After the first plasma exchange session, serum triglycerides decreased from 8100 mg/dL to 1746 mg/dL, and following the second session, this value further reduced to 543 mg/dL.
Once oral intake was resumed, fenofibrate (600 mg/day) and omega-3 (1000 mg three times daily) were started, and the patient was advised to continue these medications until delivery. By the third day of admission, her abdominal pain and tenderness had decreased significantly, and by the fifth day, she no longer reported pain, nausea, or vomiting. Figure 1 illustrates the rate of reduction in serum triglyceride levels.
Follow-up visits were scheduled biweekly until 28 weeks of gestation, weekly from 28 to 36 weeks, and twice weekly until delivery. Throughout this period, there were no episodes of recurrence. Fetal monitoring with ultrasonography and biophysical profile assessments revealed no signs of fetal growth restriction or distress. In consideration of the high-risk nature of the pregnancy and the patient’s history of infertility, an elective cesarean delivery was performed at 38 weeks of gestation.
The patient was monitored for four weeks postpartum, during which no recurrences or related complications were observed.
3. Discussion
HTG-induced pancreatitis has been reported in 1 - 7% of AP cases in pregnancy and accounts for 56% of gestational pancreatitis cases (6). Hypertriglyceridemia can be classified based on underlying etiologies as primary (genetic)—seen in congenital chylomicronemia syndrome due to lipoprotein lipase or apoprotein C-II deficiency—or secondary, due to factors such as obesity, metabolic syndrome, diabetes mellitus, pregnancy, alcohol consumption, drug use (e.g., tamoxifen, steroids, diuretics, beta-blockers, atypical antipsychotics), or idiopathic (without any predisposing factor) (7).
Pregnancy is associated with increased serum triglyceride levels, especially in the third trimester; serum triglyceride concentration in pregnant women is typically about twice that of nonpregnant women, though it rarely exceeds 300 mg/dL, much lower than the 1000 mg/dL threshold generally believed to trigger AP episodes (1).
The risk of HTG-induced AP varies by gestational age: 19% in the first trimester, 26% in the second trimester, and 53% in the third trimester (1). During the third trimester, elevated levels of estrogen and human placental lactogen (HPL), along with insulin resistance, lead to catabolic changes in lipid metabolism, including increased hepatic synthesis of very low-density lipoproteins, inhibition of hepatic lipase activity, stimulation of lipogenesis in the liver, and reduced lipoprotein lipase (LPL) activity, resulting in HTG (8).
Severe HTG in pregnancy is most commonly due to primary causes, though rare cases of hypertriglyceridemia in pregnancy without familial or genetic factors have been reported (9). The diagnostic criteria for pancreatitis in pregnant women are the same as those for non-pregnant patients and include epigastric pain, elevated serum lipase and amylase levels three times above normal, and radiologic evidence of pancreatitis (10). Ultrasonography and MRI with gadolinium are the preferred imaging modalities during pregnancy, as CT scans are typically avoided due to the fetal radiation exposure risk (2).
To reduce adverse maternal and fetal outcomes, treatment should be initiated promptly upon establishing a diagnosis of HTG-induced pancreatitis. Currently, there are no standardized guidelines for the optimal management of HTG-induced pancreatitis during pregnancy. Treatment options include supportive care (suspension of enteral feeding, hydration, and analgesia) and plasma exchange to rapidly reduce plasma triglyceride levels. Insulin infusion combined with heparin (either unfractionated or low molecular weight) has emerged over the last decade as an effective alternative, demonstrating comparable efficacy to plasma exchange in pain relief and triglyceride level reduction (2).
Insulin promotes the synthesis and activation of lipoprotein lipase (LPL), leading to chylomicron breakdown (7). A bolus dose of heparin, which has a strong affinity for the LPL binding site on capillary endothelium, competitively dissociates LPL from the endothelium into the plasma. This release results in increased free plasma LPL levels and an accelerated metabolism of plasma lipoproteins (11). However, prolonged heparin use has an opposite effect, depleting LPL on the surface of endothelial cells (7).
Plasma exchange drastically lowers lipid levels within hours, and the removal of pro-inflammatory markers and cytokines improves patient outcomes (12). Data from a non-pregnant case series indicate that after one session of plasmapheresis, triglyceride levels decreased by 66%, with levels reaching about 88% reduction after a second session. Case reports and reviews on managing HTG-induced pancreatitis using combined insulin and heparin have demonstrated a reduction rate of approximately 50% within 24 hours (2).
In our case, due to severe HTG (serum triglyceride level as high as 8100 mg/dL) and the need to rapidly reduce triglyceride levels to prevent adverse maternal and fetal outcomes, we employed both plasma exchange and combined insulin infusion with low molecular weight heparin to achieve a rapid reduction in plasma triglyceride levels. As a result, the serum triglyceride level dropped to 1746 mg/dL (77% reduction) after 24 hours and to 543 mg/dL (93% reduction) after 48 hours. The simultaneous use of these two methods was associated with a higher reduction rate, which is crucial in pregnancy to avoid complications for both the mother and fetus.
Currently, there is no established evidence on the optimal gestational age or triglyceride threshold level for elective pregnancy termination or the preferred delivery route in such cases (6). A recent study by He et al. introduced multidisciplinary team management with a lowered threshold for cesarean sections, resulting in a substantial increase in the rate of cesarean sections (from 30.8% during 2005 - 2014 to 60% in 2015 - 2019), a significant drop in fetal mortality (from 30.8% to 6.7%), and a reduction in maternal mortality (from 6% to zero) (13).
In this case, elective delivery by cesarean section was planned for the early term period (at 38 weeks of gestation) given the high-risk nature of the pregnancy, a history of infertility, and to avoid prematurity-related complications, as well as potential complications from a recurrence of HTG-induced pancreatitis if the pregnancy continued until spontaneous labor occurred.
5.1. Conclusions
In light of this case, any epigastric pain during any trimester of pregnancy should prompt consideration of acute pancreatitis, even in the absence of a personal or family history of dyslipidemia. Early diagnosis is essential to prevent severe maternal and fetal complications.