1. Background
Primary percutaneous coronary intervention (PPCI) is a proven and safe method for treating acute myocardial infarction (AMI); however, it is not without risk. Valvular heart disease is a significant complication that can develop following primary PPCI in patients with AMI. Tricuspid regurgitation (TR) and mitral regurgitation (MR) post-PCI can significantly impact the recovery and long-term prognosis of these patients, and they are a matter of concern due to their potential to increase patient morbidity and mortality. Research indicates that these complications can arise from various factors, including mechanical injury from the procedure, ischemic damage to the papillary muscles, or as a component of the systemic inflammatory response known as post-cardiac injury syndrome (1). Pericardial effusion (PE) is a prevalent complication that can occur after ST-segment elevation myocardial infarction (STEMI), with incidence rates reported between 5% and 43% (2). The presence of PE in patients with STEMI has been linked to adverse outcomes (3). However, most prior studies have focused on patients treated with fibrinolysis, and there is limited data regarding the impact of PE on long-term mortality in STEMI patients. The decreasing incidence of PE has been associated with increased use of reperfusion therapy (4). The development of PE following AMI could be attributed to several factors, including infarct size, poor hemodynamic status, and potential pericardial inflammation resulting from extensive epicardial necrosis (5).
2. Objectives
This article aims to investigate the frequency of MR, TR, aortic insufficiency (AI), and PE in patients who have experienced STEMI and are undergoing PPCI. This could help in understanding the complications associated with STEMI and the effectiveness of the intervention.
3. Methods
We conducted a prospective, single-center observational study involving STEMI patients who underwent PPCI at BouAli Medical Center, a tertiary referral educational hospital offering PPCI services, from January 2022 to March 2023. The study examined the association between PPCI and key clinical and echocardiographic parameters. Medical history variables such as diabetes mellitus (DM), hypertension (HTN), and hyperlipoproteinemia (HLP) were assessed due to their significant role in the development of coronary artery disease (CAD). Diabetes mellitus was defined as a previous diagnosis of DM, including the current use of any glucose-lowering medications and a random plasma glucose level of ≥ 200 mg/dL (6). Hypertension was defined as a systolic blood pressure of ≥ 140 mmHg or a diastolic blood pressure of ≥ 90 mmHg, while patients with stage I HTN (systolic 130 - 139 mmHg or diastolic 80 - 89 mmHg) generally did not meet criteria for pharmacological treatment (7). Patients with HTN were treated with antihypertensive medications for an average duration of 6 months. Hypertension was defined as a total cholesterol level > 240 mg/dL (8). Patients with HLP were treated with statins and other lipid-lowering medications for an average duration of 6 months. Current cigarette smoking was defined as having smoked at least 100 cigarettes during one’s lifetime and currently smoking daily or some days at the time of the survey (9). The diagnosis of STEMI was made based on established guidelines, which include a history of typical chest pain, diagnostic changes on electrocardiograms, and serial increases in cardiac biomarkers (10). Segment elevation myocardial infarction was defined by (1) presentation within 12 hours of typical symptoms onset; (2) ST-segment elevation of ≥ 1 mm in two contiguous leads on the electrocardiogram (≥ 2 mm in precordial leads); and (3) angiographic confirmation of acute coronary artery occlusion or subocclusion, indicated by a thrombolysis in myocardial infarction (TIMI) flow grade of 0 to 1.
Primary percutaneous coronary intervention was conducted in patients presenting with symptoms lasting 12 hours or less, as well as in those with persistent symptoms for 12 to 24 hours provided that the pain was consistent upon admission. All patients underwent comprehensive transthoracic echocardiography within 24 hours following revascularization. Patient records were evaluated to determine the prevalence and severity (mild, moderate, or severe) of MR, TR, AI, and PE, along with their associations with the clinical profile and key echocardiographic parameters. Family history was defined as having a first-degree relative with a documented history of early CAD, characterized by the occurrence of myocardial infarction or coronary revascularization before age 55 in male and 65 in female first-degree relatives (11).
Following PPCI, all patients underwent transthoracic echocardiography within 6 to 72 hours of coronary care unit admission performed by an echocardiography fellow. Relevant data were gathered from clinical echocardiographic examination reports. Echocardiography was conducted using the Philips Affiniti 50 Ultrasound Machine (Philips Healthcare, Andover, MA, USA) with the patient positioned in the left lateral decubitus position. Doppler measurements were obtained using 2.5 MHz and 2.5 - 3.5 MHz probes. All measurements adhered to the American Echocardiographic Association guidelines (12). To standardize volumetric comparisons, atrial and ventricular volumes were indexed to body surface area (BSA). Left ventricular ejection fraction (LVEF) was determined using the Simpson method (13). Valvular diseases were assessed through bidimensional (2D)-echocardiography in accordance with ASE/EACVI guidelines (14-16).
To evaluate the severity of TR, specific indicators were examined to identify either less-than-mild or severe regurgitation. These indicators included the color jet area (thin, small central versus large > 50% jet area), vena contract width (< 0.2 cm or 27 mm), density of the continuous Doppler jet (faint or dense and triangular), hepatic vein flow pattern (systolic dominant versus systolic reversal), annular diameter (normal versus dilated annulus with inadequate valve coaptation), and sizes of the right ventricle (RV) and right atrium (RA) (normal versus dilated). If all signs and indices were consistent, TR was classified as either less-than-mild or severe. If qualitative or semiquantitative parameters fell into an intermediate range between mild and severe, TR was defined as at least moderate to severe if the majority (five or more) of the signs and indices aligned with severe TR (17). Pericardial effusion was identified and quantified according to current guidelines (18), categorizing it as small (≤ 10 mm) or moderate-to-severe (> 10 mm), as previously suggested (19).
3.1. Statistical Analysis
All data were summarized and presented as mean ± standard deviation (SD) for continuous variables, unless otherwise specified, and as the number (percentage) of patients in each group for categorical variables. The chi-square test was utilized to calculate P-values for categorical variables. Continuous variables were compared using either the independent sample t-test or the Mann–Whitney U test. To assess correlations between echocardiographic parameters and other variables in the patient population, Pearson’s coefficient and Spearman’s rho were employed. A two-tailed P-value of < 0.05 was regarded as statistically significant for all analyses. The statistical analyses were conducted using version 27 of the IBM Statistical Package for the Social Sciences (SPSS, IBM Corp, Armonk, NY, USA).
4. Results
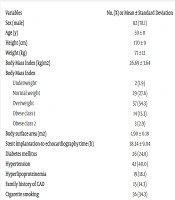
The study included 105 participants, with a male predominance of 78.1% (82 patients). The mean age of the participants was 59 years, with a standard deviation (SD) of 11 years. The mean Body Mass Index (BMI) was 26.69 (± 3.64) kg/m², with the majority of participants being overweight (57 patients, 54.3%). Regarding medical history, 26 patients (24.8%) had diabetes mellitus (DM), and 42 patients (40.0%) had HTN. Hyperlipoproteinemia and a family history of CAD were less common, being present in 19 (18.1%) and 15 (14.3%) participants, respectively. Cigarette smoking was reported by 34.3% of participants (36 patients).
The mean ejection fraction (EF) was 38.36% ± 9.05%. The left ventricular end-systolic and end-diastolic diameters were 36 ± 8 mm and 56 ± 7 mm, respectively. The deceleration time (DT) was 178.52 ± 61.54 milliseconds. The end-systolic and end-diastolic volumes were 55.51 ± 21.33 mm³ and 89.5 ± 26.1 mm³, respectively. The mean pulmonary artery pressure (PAP) was 30.03 ± 6.68 mmHg. The tricuspid regurgitant velocity (TRV) and regurgitation gradient (TRG) were 2.39 ± 0.27 m/s and 23.92 ± 5.33 mmHg, respectively. The right ventricular peak systolic myocardial velocity (RVSm) was 9.61 ± 1.79 cm/s.
Mitral regurgitation (MR) was mostly mild (88 patients, 83.8%), with moderate cases accounting for 14.3% (15 patients). Tricuspid regurgitation (TR) followed a similar pattern, with 85.7% of cases classified as mild (90 patients). Aortic insufficiency (AI) was predominantly absent or mild, with no severe cases reported, and only one patient had moderate AI. Pericardial effusion was absent in 89 patients (84.8%) and was mild in 16 patients (15.2%). Table 1 presents the baseline characteristics of the study participants.
| Variables | No. (%) or Mean ± Standard Deviation |
|---|---|
| Sex (male) | 82 (78.1) |
| Age (y) | 59 ± 11 |
| Height (cm) | 170 ± 9 |
| Weight (kg) | 77 ± 12 |
| Body Mass Index (kg/m2) | 26.69 ± 3.64 |
| Body Mass Index | |
| Underweight | 2 (1.9) |
| Normal weight | 29 (27.6) |
| Overweight | 57 (54.3) |
| Obese class 1 | 14 (13.3) |
| Obese class 2 | 3 (2.9) |
| Body surface area (m2) | 1.90 ± 0.18 |
| Stent implantation-to echocardiography time (h) | 38.34 ± 9.04 |
| Diabetes mellitus | 26 (24.8) |
| Hypertension | 42 (40.0) |
| Hyperlipoproteinemia | 19 (18.1) |
| Family history of CAD | 15 (14.3) |
| Cigarette smoking | 36 (34.3) |
| Coronary vessel involvement | |
| Left anterior descending | 50 (47.6) |
| Left circumflex artery | 14 (13.3) |
| Ramus circumflex artery | 41 (39.0) |
| Simpson ejection fraction | 38.36 ± 9.05 |
| Left ventricular end‑diastolic diameter (mm) | 56 ± 7 |
| Left ventricular end‑systolic diameter (mm) | 36 ± 8 |
| Deceleration time (ms) | 178.52 ± 61.54 |
| End-diastolic volume (mL) | 89.5 ± 26.1 |
| End-systolic volume (mL) | 55.51 ± 21.33 |
| Mean pulmonary artery pressure (mmHg) | 30.03 ± 6.68 |
| Early diastolic filling velocity (m/s) | 0.70 ± 0.23 |
| Tricuspid regurgitant velocity (m/s) | 2.39 ± 0.27 |
| Tricuspid regurgitation gradient (mm Hg) | 23.92 ± 5.33 |
| Right ventricular peak systolic myocardial velocity (m/s) | 9.61 ± 1.79 |
| Mitral Regurgitation | |
| No | 1 (1.0) |
| Mild | 88 (83.8) |
| Moderate | 15 (14.3) |
| Severe | 1 (1.0) |
| Tricuspid regurgitation | |
| No | 4 (3.8) |
| Mild | 90 (85.7) |
| Moderate | 10 (9.5) |
| Severe | 1 (1.0) |
| Aortic insufficiency | |
| No | 88 (83.8) |
| Mild | 16 (15.2) |
| Moderate | 1 (1.0) |
| Severe | 0 (0) |
| Pericardial effusion | |
| No | 89 (84.8) |
| Mild | 16 (15.2) |
| Moderate and sever | 0 (0) |
Table 2 summarizes the frequency and severity of MR, TR, AI, and PE among the studied patients.
| Variables | MR | P-Value | TR | P-Value | AI | P-Value | PE | P-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Mild | Moderate | Mild | Mild | |||||
| Male | 73 (83) | 8 (53.3) | 0.017 b | 73 (81.1) | 5 (50) | 0.044 b | 10 (62.5) | 0.103 b | 10 (62.5) | 0.099 |
| Age (y) | 58 ± 11 | 63 ± 10 | 0.112 | 58 ± 11 | 69 ± 11 | 0.012 b | 64 ± 12 | 0.034 b | 58 ± 14 | 0.791 |
| Height (m) | 171 ± 8 | 163 ± 9 | < 0.01 b | 171 ± 9 | 166 ± 8 | 0.210 | 167 ± 11 | 0.130 | 169 ± 12 | 0.539 |
| Weight (kg) | 78 ± 12 | 70 ± 11 | 0.020 b | 78 ± 12 | 70 ± 10 | 0.138 | 72 ± 12 | 0.060 | 77 ± 18 | 0.889 |
| BMI (kg/m2) | 26.7 ± 3.8 | 26.4 ± 2.9 | 0.816 | 26.8 ± 3.7 | 25.4 ± 3.0 | 0.493 | 25.9 ± 4.2 | 0.321 | 26.9 ± 5.3 | 0.848 |
| BSA (m2) | 1.9 ± 0.18 | 1.8 ± 0.2 | 0.005 b | 1.9 ± 0.2 | 1.8 ± 0.2 | 0.111 | 1.8 ± 0.2 | 0.049 b | 1.9 ± 0.3 | 0.680 |
| DM | 20 (22.7) | 5 (33.3) | 0.279 | 24 (26.7) | 1 (10) | 0.513 | 5 (31.3) | 0.365 | 3 (18.8) | 0.400 |
| HTN | 31 (35.2) | 9 (60) | 0.064 | 34 (37.8) | 4 (40) | 0.329 | 8 (50) | 0.394 | 7 (43.8) | 0.739 |
| HLP | 10 (11.4) | 7 (46.7) | 0.003 b | 15 (16.7) | 2 (20) | 0.238 | 2 (12.5) | 0.403 | 3 (18.8) | 0.589 |
| CAD FH | 13 (14.8) | 2 (13.3) | 0.623 | 15 (16.7) | 0 (0) | 0.256 | 1 (6.3) | 0.282 | 4 (25) | 0.170 |
| CS | 33 (37.5) | 2 (13.3) | 0.068 | 32 (35.6) | 3 (30) | 0.864 | 2 (12.5) | 0.043 b | 5 (31.3) | 0.781 |
Abbreviations: AI, aortic insufficiency; BMI, Body Mass Index; CAD, coronary artery disease; MR, Mitral regurgitation; TR, tricuspid regurgitation; BSA, body surface area (BSA); DM, diabetes mellitus; HTN, hypertension; HLP, hyperlipoproteinemia; CAD FH, family history of coronary artery disease; CS, cigarette smoking; PE, pericardial effusion; PPCI, primary percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
a Categorical variables were expressed as percentages, while continuous variables were reported as mean ± standard deviation.
b P ≤ 0.05 was considered statistically significant.
The results indicated that MR was significantly associated with sex, HLP, and BSA. Male patients showed a higher prevalence of MR compared to female patients (P < 0.001). Additionally, patients with HLP exhibited lower MR levels than those without the condition (P < 0.001). Moreover, MR demonstrated a negative correlation with BSA (r = -0.27, P < 0.001). Tricuspid regurgitation was significantly associated with both sex and age; male patients exhibited higher TR levels compared to female patients (P = 0.042). Additionally, TR showed a positive correlation with age (r = 0.2, P < 0.05). AI was significantly associated with cigarette smoking, with smokers displaying lower AI levels compared to non-smokers (P = 0.043). The relationships between these variables are summarized in Table 3.
| Spearman's Rho | MR | TR | AI | PE |
|---|---|---|---|---|
| Sex | -0.255 a | -0.244 b | -0.158 | -0.160 |
| Diabetes mellitus | 0.087 | -0.094 | 0.062 | -0.059 |
| Hypertension | 0.179 | -0.071 | 0.084 | 0.032 |
| Hyperlipoproteinemia | 0.335 a | -0.071 | -0.064 | 0.007 |
| Cigarette smoking | -0.180 | -0.006 | -0.198 b | -0.027 |
| Correlation coefficient Pearson | ||||
| Age (y) | 0.159 | 0.198 b | 0.187 | -0.045 |
| Body Mass Index (kg/m2) | -0.020 | -0.121 | -0.154 | 0.079 |
| Body surface area (m2) | -0.274 a | -0.161 | -0.159 | -0.002 |
Abbreviations: AI, aortic insufficiency; MR, Mitral regurgitation; TR, Tricuspid regurgitation; PE, pericardial effusion; PPCI, primary percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
a P < 0.01.
b P < 0.05.
In this study, only mild cases were followed up, while moderate to severe cases were managed according to the physician’s clinical judgment. No instances were reported of accompanying conditions or side effects following intervention that led to new symptoms requiring further interventions or additional drug treatments. The treatment approach was individualized based on the severity of the condition and the recommendations of the treating physician.
5. Discussion
Overall, the findings of this study add to the expanding body of evidence regarding PPCI. We investigated the prevalence of postprocedural conditions (MR, TR, and PE) in this patient population. In addition, the demographic and clinical characteristics and other postprocedural echocardiographic findings of these patients were reported. The findings suggest that mild regurgitation is common, with moderate cases being less frequent and severe cases rare.
Our study primarily observed mild MR, accounting for 83.8% of cases. Moderate cases made up 14.3%, while severe cases were rare at 1%. We found a significant association between MR, sex, and HLP. Male patients exhibited higher MR than female patients, and patients with HLP had lower MR than those without. Mild to moderate MR is prevalent among patients with AMI, affecting between 13% and 45% of individuals (20, 21). Most cases of MR are transient, asymptomatic, and benign. However, severe MR resulting from papillary muscle rupture can be life-threatening. The GUSTO J trial found that MR is a predictor of poor prognosis, with even mild to moderate MR linked to increased mortality. Patients with MR after AMI have a poorer prognosis than those without MR (22, 23). Nikolsky et al. found that among patients with STEMI undergoing PPCI, 10% had mild MR and 3% had moderate to severe MR (24). A single-center retrospective observational study revealed that 29% of patients undergoing PCI had MR. The reported prevalence of MR lies between the findings of the two most recent studies focused exclusively on STEMI patients. In line with previous research, patients with MR faced significantly higher mortality rates, with outcomes diverging within the first year and continuing over an average of 3 years. Advancing age and reduced LVEF are strong independent predictors of mortality in these patients.
Several mechanisms may contribute to MR. These factors include dilation of the mitral valve annulus due to left ventricular dilation, partial or complete rupture of the chordae or papillary muscle, and dysfunction of the papillary muscle associated with ischemic regional wall-motion abnormalities near the posterior papillary muscle insertion (25). The prevalence of ischemic MR, as determined by Doppler echocardiography, varies widely from 8% to 74% and from 1.6% to 9% when determined by angiography (26). This wide range is due to the variability in studies estimating the prevalence of acute MR, with differences in design, timing of imaging, severity of MR, and the techniques used. Ischemic MR has both short-term and long-term prognostic significance.
Our study found that TR was primarily mild in 85.7% of the cases, moderate in 9.5%, and severe in 1%. We also discovered a significant correlation between TR, sex, and age. Male patients exhibited higher TR than female patients, and TR increased with age. Although TR is a common finding on echocardiograms (27), it is often overlooked as a clinically insignificant condition. Assessing the clinical impact and outcomes of TR is challenging due to its heterogeneity and association with various comorbidities. Key studies suggest that untreated TR is linked to increased mortality and cardiac events (28, 29). Additionally, TR has been associated with adverse cardiovascular outcomes. For example, Sadeh et al. found that in the STEMI patient population, TR was linked to increased mortality, even after adjusting for demographic, clinical, and echocardiographic parameters (29).
In our research, AI was primarily absent or mild, and no severe cases were reported. We also found a significant correlation between AI and cigarette smoking. Patients who smoked exhibited lower AI than non-smokers. Acute iatrogenic AI is an extremely rare mechanical complication of PPCI with an incidence of approximately 1 in 10,000 (30). The reported prevalence of aortic valve injury during coronary angiography (CAG) and PCI ranges from 0.008% to 0.20% (31). In the past five years, only four case reports have documented acute AI following coronary angiography or PCI. This condition typically arises from multiple attempts with various catheters to access the ostium of the right coronary artery, but it can also occur when accessing the left coronary artery ostium (32).
In our study, PE was predominantly absent (84.8%) or mild (15.2%), with no instances of moderate or severe cases. Pericardial effusion is commonly observed in the acute phase of STEMI (2, 4). A prospective cohort study reported that post-cardiac injury was the primary cause of pericarditis, accounting for 21% of patients (1). Early pericarditis following AMI occurs in approximately 10% of cases, typically developing 24 to 96 h after MI (25). Risk factors for PE have been identified, with moderate PE associated with increased mortality (3). However, previous studies are outdated, and many patients have received fibrinolytic treatment. In the current era of PPCI, the incidence of PE remains under-studied (4). In acute STEMI patients treated with PCI, the occurrence of PE was not independently linked to increased short- and long-term mortality. A recent study examining the relationship between PE and survival in a contemporary cohort of STEMI patients treated with PPCI found that PE was present in 14% of these patients. While PE is more prevalent in those with high-risk characteristics, such as larger infarct sizes, it does not correlate with higher mortality rates in either the short or long term (4). These findings are significant for two reasons: First, they enhance our understanding of the impact of PE following STEMI treatment in the PPCI era regarding both short- and long-term prognoses. Second, they indicate that PE has a minor role in prognostic stratification in contemporary STEMI treatment. Although PE does not significantly influence survival outcomes, its early detection may still be crucial, as severe complications like free wall rupture are more likely to occur in patients who develop PE shortly after reperfusion.
We acknowledge several limitations of our study. Conducted at a single center, the findings may have limited generalizability. There was a lack of information regarding the presence of PE and valve issues prior to the occurrence of AMI. Additionally, it was challenging to accurately attribute existing symptoms in patients to the identified valve problems on echocardiography, especially in cases of mild to moderate severity. Furthermore, the frequency and severity of regional wall motion abnormalities (RWMA) were not recorded, as this variable was not included in the initial study design.
5.1. Conclusions
The study concluded that MR and TR were relatively common complications following PPCI in patients with STEMI. However, they were predominantly mild, which may reflect the transient effects of the intervention or the underlying cardiac conditions. The absence of moderate and severe PE and AI was a positive finding, indicating that significant complications in this area are uncommon. Future research endeavors should focus on exploring interventions aimed at mitigating the occurrence and severity of these conditions. Additionally, investigating the impact of regional wall motion abnormalities (RWMA) on the studied conditions should be a key consideration in further research efforts.