1. Background
Attention to the indoor air quality of hospitals due to the dispersal of airborne bioaerosols is important (1). Fungi and bacteria constitute a wide range of microorganisms in the indoor and outdoor environment of hospitals (2). Indoor air quality, the number of hospital personnel, design of patient rooms, disinfection methods, type of ventilation, temperature, and humidity are effective factors in the amount and variety of pollution in the hospital environments (3, 4). Inhalation of bioaerosols can cause adverse effects on human health including chronic infections, allergic reactions, inflammation, and respiratory disease (5).
The spread of fungal spores in outdoor and indoor environments of hospitals is important as a potential contributor to nosocomial fungal infections. In recent years, fungal infections in hospitalized patients have increased (6, 7). Aspergillus, Cladosporium, Penicillium, and Alternaria species have been identified as the most frequent fungi in indoor and outdoor hospital environments. Invasive fungal infections (IFI), especially as a result of inhaling Aspergillus spores, are dangerous to the health of hospitalized and immunocompromised patients (8). Evidence indicates that fungal contamination in indoor and outdoor air of hospitals challenges the treatment of fungal infections (9). There have been reports of the resistance of environmental A. fumigatus isolates to medical triazoles in the indoor environment of hospitals, patient rooms, soil, and compost (10). Therefore, it is important to identify nosocomial infection reservoirs and to improve air quality in the hospital environments (11, 12).
2. Objectives
The aim of this study was to evaluate the fungal diversity and determine the concentrations of airborne fungi by active sampling in two educational hospitals (A and B) of Qazvin city, Iran.
3. Methods
3.1. Air Sampling
In the present cross-sectional descriptive study, the air fungal contamination load was carried out during six months (winter and spring) in different wards (emergency A/B), infectious ward (male/female A), internal ward (male/female B), intensive care unit (ICU) (A/B) and operating room (A/B) of two educational hospitals in Qazvin city, Iran. Air sampling was performed according to NIOSH-0800 instructions using the Quick Take-30 pump with an air flow rate of 28.3 L/min for 2 minutes at a distance of 120 - 150 cm from the floor and 1 meter from the walls and barriers. The air sampling was carried out during the week between 8 am and 3 pm. In order to detect fungal spores, petri dishes with a diameter of 9 cm containing sabouraud dextrose agar (SDA) (Merck, Germany) supplemented with chloramphenicol (0.5 mg/mL) (prevent the growth of bacteria) were used. Sampling time was performed in different wards from 8:00 am to 14:00 pm. After the sampling, the plates were transferred to the clinical mycology laboratory of the Qazvin University of Medical Sciences.
3.2. Isolation and Identification of Fungi
The SDA plates were incubated at 27 - 30°C for 7 to 10 days. The filamentous fungi and yeasts were identified at the genus or species level by standard mycological techniques based on morphological characteristics (macroscopic and microscopic) by lactophenol cotton blue (LCB) mounts and slide culture.
The mean number of fungal colonies was calculated using the following formula was presented as colony-forming units per cubic meter (CFU/m3) of air.
In this formula, C is the number of colonies, T is the sampling time (min), and V is the sampling air flow (L/min).
3.3. Statistical Analysis
The collected data were analyzed using SPSS software version 22 and independent sample t-test and one-way ANOVA tests. Significance level was considered at P < 0.05.
4. Results
A total of 165 air samples were collected and cultivated from ten wards of two hospitals indoor. During the study period, a total number of 83.12 CFU/m3 and 81.37 CFU/m3 were isolated from hospitals A and B, respectively. Among the studied wards, the highest air fungal contamination load was related to the emergency ward (108 CFU/m3), followed by infectious ward (84.6 CFU/m3), internal ward (73 CFU/m3), intensive care (65.6 CFU/m3), and operating room (34.2 CFU/m3) (Table 1).
| Sampling Sites | Fungal Aerosols | ||
|---|---|---|---|
| Mean ± SD (CFU/m3) | Min-Max | P-Value | |
| Emergency | 108 ± 59.5 | 35.7 - 267.8 | < 0.05 |
| Infectious | 84.6 ± 39.9 | 17.8 - 178.5 | |
| Internal | 73 ± 29.2 | 17.8 - 142.8 | |
| ICU | 65.6 ± 26.3 | 17.8 - 125 | |
| Operating room | 34.2 ± 11.9 | 17.8 - 53.5 | |
Mean Number of Fungi (CFU/m3) in Air Samples Collected from Various Wards
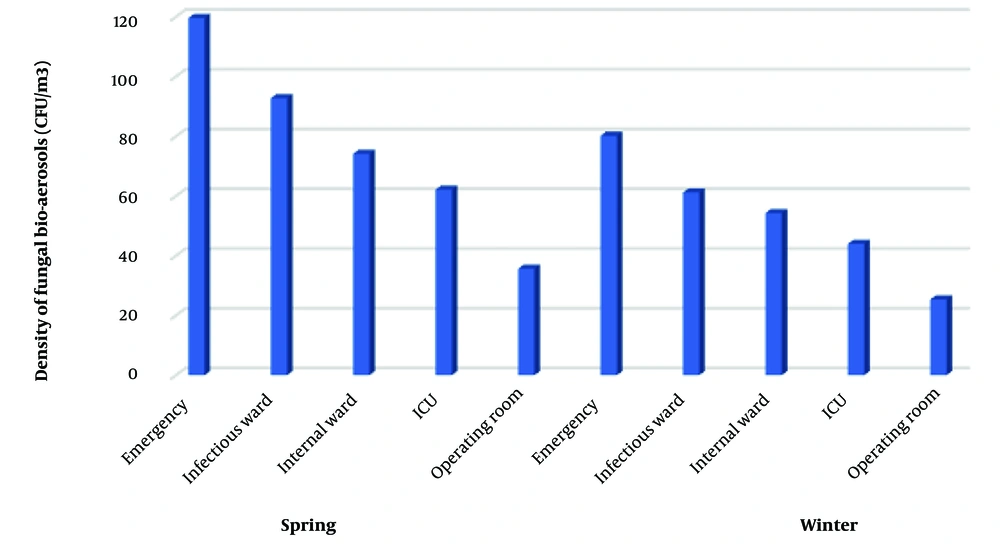
There was a significant difference in the air fungal contamination load between various wards of hospitals (P < 0.05). The highest mean fungal contamination in the emergency ward of two hospitals in spring and winter was 119.6 CFU/m3 and 85.6 CFU/m3, respectively (Figure 1). The average number of fungi (CFU/m3) in spring and winter was 87.13 CFU/m3 and 74.7 CFU/m3, respectively. There was a significant difference in the air fungal contamination load CFU/m3) and seasons (P < 0.05). A total of 1021 fungal colonies belonging to 13 genera were isolated from all wards. The results indicated among the different isolated fungal species, Aspergillus ssp. (n = 310, 30.3%) was the most prominent isolated genus followed by Cladosporium ssp. (n = 218, 21.3%), Penicillium spp. (n = 193, 19%), Alternaria spp. (n = 136, 13.3%), Mucor spp. (n = 66, 6.5%), and other fungi (n = 98, 9.5%) (Table 2).
| Fungi | Emergency | Infectious | Internal | ICU | Operating | Total |
|---|---|---|---|---|---|---|
| Aspergillus spp. | 101 (26.5) | 72 (30.3) | 72 (31.1) | 58 (39) | 7 (30.4) | 310 (30.3) |
| Cladosporium spp. | 87 (22.8) | 58 (24.4) | 47 (20.3) | 26 (17.5) | - | 218 (21.3) |
| Penicillium spp. | 65 (17) | 48 (20.2) | 45 (19.5) | 34 (22.8) | 1 (4.3) | 193 (19) |
| Alternaria spp. | 37 (9.7) | 30 (12.6) | 33 (14.3) | 21 (14) | 15 (65.2) | 136 (13.3) |
| Mucor spp. | 35 (9.2) | 11 (4.6) | 19 (8.2) | 1 (0.67) | - | 66 (6.5) |
| Paecilomyces spp. | 13 (3.4) | 2 (0.84) | 2 (0.86) | - | - | 17 (1.6) |
| Fusarium spp. | 5 (1.3) | 1 (0.42) | 2 (0.86) | 2 (1.3) | - | 10 (0.97) |
| Rhizopus spp. | 9 (2.3) | 2 (0.84) | - | - | - | 11 (1.07) |
| Sterilehyphae spp. | 17 (4.4) | 3 (1.2) | 4 (1.7) | 3 (2) | - | 27 (2.6) |
| Acremonium spp. | 4 (1.04) | - | 3 (1.3) | - | - | 7 (0.68) |
| Drechslera spp. | - | 3 (1.2) | 4 (1.7) | - | - | 7 (0.68) |
| Curvularia spp. | 3 (0.78) | 5 (2.1) | - | - | - | 8 (0.78) |
| Yeast spp. | 5 (1.3) | 2 (0.84) | - | 4 (2.6) | - | 11 (1.07) |
| Total | 381 (37.3) | 237 (23.2) | 231 (22.6) | 149 (14.6) | 23 (2.2) | 1021 (100) |
Distribution of Fungi Isolated from Different Departments of the Two Hospitals a
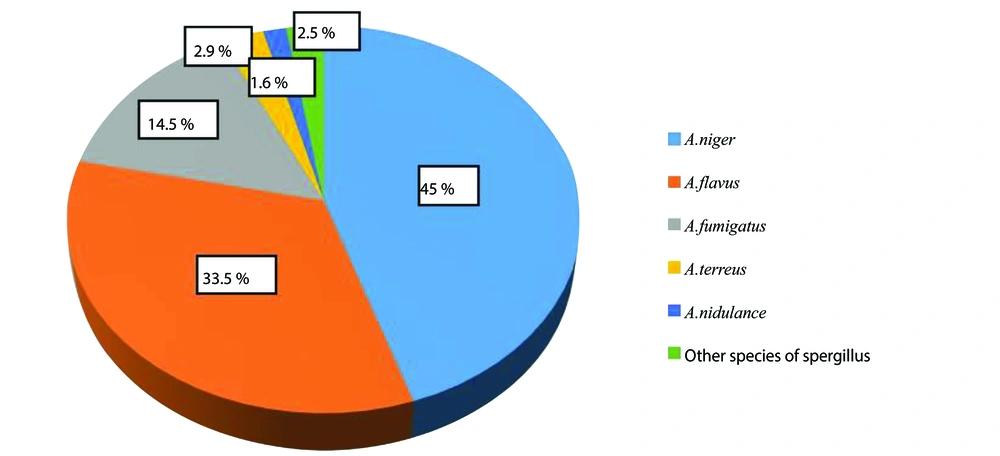
Almost all studied species were found in indoor air samples of both hospitals. Among isolates of Aspergillus, A. niger (n = 139, 45%), A. flavus (n = 104, 33.5%), and A. fumigatus (n = 45, 14.5%), A. terreus (n = 9, 2.9%), A.nidulans (n = 5, 1.6%), and other Aspergillus spp. (n = 8, 2.5%) were the commonest species (Figure 2). The highest and lowest rates of isolation of Aspergillus species were from the emergency ward and operating room, respectively.
5. Discussion
The results indicated different degrees of fungal contamination in the indoor air of different wards, particularly in the emergency and infectious wards. According to World Health Organization (WHO) guidelines, relatively low limits of 100 CFU/m3 for bacteria and 50 CFU/m3 for fungi are recommended in hospital air; however, many health centers cannot provide this limit (13). The concentration of airborne fungi ranged from 17.8 to 267.8 CFU/m3 in the present study. Moreover, among the studied wards, the highest fungal contamination was related to the emergency ward (108 CFU/m3), infectious ward (84.6 CFU/m3), internal ward (73 CFU/m3), and intensive care unit (65.6 CFU/m3), respectively, which was higher than WHO standards. The lowest level of airborne fungal was observed in the operating room (34.2 CFU/m3). Increased the number of personnel and patients and long-term hospitalization of patients can facilitate increased biological load. On the other hand, inadequate ventilation can exacerbate the accumulation of airborne spores. Therefore, the World Health Organization's recommended limits for airborne fungi are crucial in maintaining a safe environment for patients, especially those with compromised immune systems. Montazeri et al. reported that the level of fungal contamination was 0 - 1047 CFU/m3 (14). In this study, the derm ward (110 CFU/m3) showed the highest level of fungal contamination compared to other wards. Kiasat et al. recorded the total mean number of fungal in the indoor environment of hospitals in the city of Ahvaz as 195.59 CFU/m3. In this study, the highest number of fungi were related to surgical ward (446 CFU/m3) (15). Various factors such as climatic conditions and geographical changes, construction activities, outdoor air intake, the efficiency of the ventilation systems, and the number of personnel can significantly increase the load of fungal contamination in the indoor air of different wards (16, 17). Previous studies show seasonal changes in the concentration of fungi in the air indoor and outdoor the hospitals (18, 19). In our study, the average fungal density in spring (April to June) was more than in winter (January to March), so a significant difference was shown between CFU/m3 and season (P < 0.05). Kabir et al. stated that the indoor air pollution of hospitals is affected by the sampling seasons (20). Studies have reported the concentration of airborne pollutants in the indoor air in the summer due to the entry of outside air and the absence of fresh air in winter due to the closed entrance doors (3, 21). According to the results of the studies, the reason for the increase in fungal contamination in the spring can be related to the increase in temperature and relative humidity. Therefore, with the beginning of the spring season, more attention should be paid to the purification of the air entering the wards. In most studies, three genera of Aspergillus, Penicillium, and Cladosporium have been reported as the most common fungal agents isolated from hospital air (9, 22). Among filamentous fungi, the most prevalent fungal isolated from the air hospitals was Aspergillus species (30.3%) followed by Cladosporium spp. (21.3%), Penicillium spp. (19%), Alternaria spp. (13.3%), Mucor spp. (6.5%) and other fungi (9.5%). Among isolates of Aspergillus, A. niger complex (45%) showed the highest frequency. The fungal species isolated in the present study aligns with the results of many studies. Ghazanfari et al. showed that Aspergillus spp. (39.5%), Cladosporium spp. (16.6%), and Penicillium spp. (10.4%) were the most isolates identified from hospital air (23). Kiasat et al. revealed that Cladosporium spp. (35.3%), Aspergillus spp. (15.1%), and Penicillium spp. (12.1%) were the most common fungal agents isolated from hospital air, respectively (15). In another study by Ziaee et al., Aspergillus spp. (16.4%), Penicillium spp. (15.7%), and Cladosporium spp. (13.14%) species were the most common isolates from the indoor and outdoor air (24). The presence of Aspergillus species in the indoor air of the hospital is a significant concern because Aspergillus species are associated with allergic reactions, invasive aspergillosis, and mycotoxin production, which is dangerous in patients, especially in immunocompromised patients (25, 26). The construction and renovation activities close to hospital sites is one of the factors that increase the dispersion of Aspergillus spores in the hospital environment. This issue can be dangerous for hospitalized patients with immune system deficiency (27, 28). In addition, other fungal spores isolated from the indoor air of the hospital may play a role in causing allergies and respiratory infections in healthy individuals and patients with immunodeficiency (2, 29). Studies show that airborne transmission of hospital infections is about 10 - 20%. The indoor air quality of the hospital is critical to the health status of healthcare workers and patients. Therefore, regular monitoring of indoor air quality is important in healthcare centers (30).
5.1. Conclusions
The distribution of fungal spores in the indoor air of hospital wards can serve a potential reservoir for the outbreak of nosocomial infections. The level of fungal contamination in different wards, exceeding WHO guidelines, underscores the urgent need to improve air quality measures in high-risk wards. The significant presence of fungal spores in hospital air highlights an urgent need for comprehensive air quality management strategies to prevent nosocomial infections. Upgrading the ventilation system, restoration of the building, regular disinfection of floors, surfaces, and medical equipment and restricting commuting are highly recommended.