1. Background
Multisystem inflammatory syndrome in children (MIS-C) is defined as a clinically serious condition requiring hospitalization, involving fever, multi-system organ dysfunction, and an increase in inflammatory biomarkers (1). A case of MIS-C is an individual younger than 21 years old, presenting with documented fever (higher than 38°C), appearing severely ill and requiring hospitalization, with multisystem involvement of at least two organs (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, or being in shock), C-reactive protein (CRP) >3.0 mg/dL, and detection of SARS-CoV-2 nucleic acid/antigen up to 60 days prior to or during hospitalization or in a postmortem specimen, OR detection of antibody associated with the current illness, OR close contact with a confirmed/probable COVID-19 case in the 60 days prior to hospitalization. This case definition was approved and announced by the Council of State and Territorial Epidemiologists (CSTE) and the Centers for Disease Control and Prevention (CDC) for MIS-C in December 2022 (2, 3).
Some studies suggest that MIS-C prevalence typically surges 3 to 6 weeks after the peak of COVID-19 in a geographic region (4). Based on the study by Alsaied et al., MIS-C occurs in clusters, typically around four weeks after the peak incidence of COVID-19 in heavily affected regions (5). The heart is the major target organ involved in MIS-C. Cardiac involvements include left ventricular dysfunction, coronary artery dilatation or aneurysm, arrhythmias, conduction abnormalities, and cardiogenic shock. Severe cases can present as vasodilatation or cardiogenic shock requiring fluid resuscitation, inotropic support, and in the most severe cases, mechanical ventilation and extracorporeal membrane oxygenation (5, 6).
Laboratory findings are highly valuable for confirming the diagnosis. According to the results of a study by Feldstein et al., most of their patients had four or more laboratory biomarkers indicating inflammation, including an elevated erythrocyte sedimentation rate or CRP level, lymphocytopenia, neutrophilia, elevated ferritin level, hypoalbuminemia, elevated alanine aminotransferase level, anemia, thrombocytopenia, an elevated D-dimer level, prolonged international normalized ratio, or elevated fibrinogen level (7).
2. Objectives
This study was carried out to assess the clinical presentations, laboratory findings, and cardiac manifestations of MIS-C, as well as cardiac involvement follow-up in a tertiary pediatric hospital.
3. Methods
This cross-sectional study was conducted in a tertiary pediatric hospital in Iran from March 2020 to December 2022. We retrospectively analyzed all admitted patients diagnosed with MIS-C according to the CDC case definition, as mentioned above (2). Thus, this constituted the inclusion criteria, and the presence of congenital heart disease was the exclusion criteria for the cases. Without considering any formula for sample size, all referred cases during the aforementioned dates who met the criteria for MIS-C were included in our study (total sampling). Given the rarity of MIS-C and the need to capture all eligible cases in our referral center, a formal sample size calculation was not performed, as total sampling was deemed appropriate to maximize study inclusivity. However, this approach may limit statistical power for detecting smaller effect sizes and could affect the generalizability of findings to broader populations, as discussed in the Limitations section.
To obtain the incidence rate of MIS-C among all children affected by COVID-19 during the period of our study, we requested the related statistical data from the province of Qazvin, from the deputy for health at Qazvin University of Medical Sciences and Health Services, through which we determined the incidence. Definitive confirmation of COVID-19 infection for inclusion in the study was performed by a pediatric cardiologist in accordance with CDC criteria.
For all patients, demographic data, clinical presentations, and laboratory findings (including white blood cells, neutrophils, lymphocytes, CRP, platelet count, hemoglobin and hematocrit, D-dimer, and troponin I) were measured and recorded. A questionnaire was designed containing demographic data of the cases and all variables of the study. All steps of data collection, physical examinations, laboratory result follow-ups, and data registration in the relevant questionnaire were performed by a pediatric cardiologist and a pediatric assistant. The sources of data were the parents and, in older cases, the children themselves. Laboratory tests were performed in the laboratory department of Qazvin Children’s Hospital, and final confirmation of COVID-19 for all cases was received from the reference laboratory designated by the Iranian Ministry of Health.
The normal range of leukocyte count was considered 4,500 to 10,000/mm3, with values less or more than this range considered as leukopenia and leukocytosis, respectively. Regarding the differences in WBC counts and age-related differences in leukocyte differentiation, absolute neutrophil counts higher than 8,500/mm3 and 7,500/mm3 were considered neutrophilia in cases younger than 5 years and those equal to or older than 5 years, respectively (8, 9). Ignoring age differences, an absolute lymphocyte count less than 1,500/mm3 was known as lymphopenia, and a count greater than this was considered normal (10). A hemoglobin level higher than 12 g/dL was considered normal, and levels less than this were considered undesirable (11). The normal range of platelets was considered 150,000 to 450,000/mm3, with values less or more than this range considered as thrombocytopenia and thrombocytosis, respectively (12). The CRP levels higher than 3 mg/L, troponin I levels higher than 0.04 ng/mL, and D-dimer levels more than 500 ng/mL were considered abnormal according to laboratory reference values.
Cardiac assessment included electrocardiography (ECG) and echocardiography, which were performed for all cases, and the related results were recorded. Cardiac function was assessed using a transthoracic echocardiogram device (Sonosite M-Turbo), through which the left ventricular function was calculated according to M-mode measurement (13). Left ventricular function was classified as normal function [ejection fraction (EF) ≥ 55%], mild dysfunction (EF 41 - 54%), moderate dysfunction (EF 31 - 40%), and severe dysfunction (EF ≤ 30%) (14). Left ventricular diastolic function was assessed by flow Doppler pattern across the mitral valve. LV diastolic function was considered dysfunctional if E/A <1 or E/A >2, and normal if E/A = 1-2. E and A waves represent early and late diastolic filling, respectively (15). We classified coronary artery anomalies based on the American Heart Association Z-score classification: Dilatation (Z-score 2 - 2.5), small aneurysm (Z-score 2.5 - 5), medium aneurysm (Z-score 5 - 10), and giant aneurysm (Z-score >10) (16). The degree of pericardial effusion is classified as small or mild if < 10 mm (< 5 mm equals 50 - 100 mL and 5 - 10 mm equals 100 - 250 mL), moderate if 10 - 20 mm (equals 250 - 500 mL), and large if > 20 mm (> 500 mL) (17). Furthermore, valvular assessment of the heart was conducted to detect the probability of regurgitation. Echocardiography was repeated after intravenous immunoglobulin (IVIG) therapy for all patients with cardiac involvement and for some cases during hospitalization if necessary. Cardiac assessments, including ECG and echocardiography, were performed at intervals for a year.
3.1. Statistical Analysis
Quantitative findings were expressed as qualitative variables and described as numbers and percentages, which were analyzed using SPSS-25 and the chi-square test. Chi-square tests were chosen due to the categorical nature of variables and sufficient expected frequencies in the sample. Cases with missing data were excluded from specific analyses to ensure the accuracy and robustness of the results. A P-value of less than 0.05 was considered a significant difference.
3.2. Ethical Consideration
This study was ethically approved by the Human Research Ethics Committee at Qazvin University of Medical Sciences and Health Services (I.R. Iran) with the approval code IR.QUMS.REC.1400.090. Informed consent was obtained from parents or legal guardians of all participants, as required for retrospective data collection involving human subjects.
4. Results
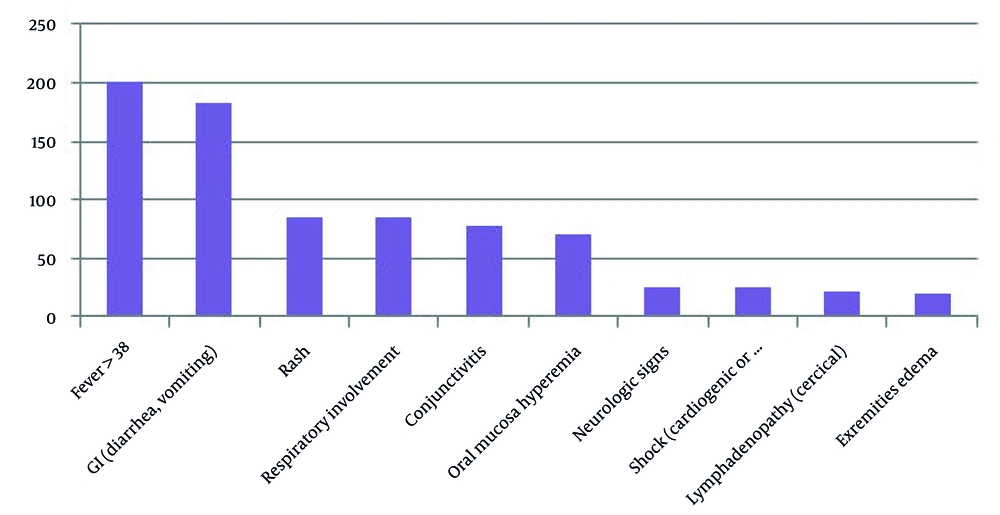
In this study, we identified 200 patients with MIS-C (108 boys and 92 girls), with a median age of 4 years, ranging from 6 months to 12 years old. None of the patients had congenital heart disease, and 64 (32%) patients had a positive PCR for COVID-19. According to the statistical data received from the Provincial Health Center, there were 11,494 cases of children up to twelve years old with COVID-19 involvement; therefore, the incidence of MIS-C was 1.74 percent. Clinical presentations of MIS-C patients are shown in Figure 1.
Laboratory findings in MIS-C patients were classified into two groups: Those with and without cardiac involvement (Table 1).
| Laboratory Findings | Cardiac Involvement | Normal Heart | K2 | P-Value |
|---|---|---|---|---|
| White blood cell (n/mm3) | 0.001709 | |||
| Normal (4500 - 10,000) | 40 (20) | 83 (41.5) | 12.7439 | |
| Leukocytosis (> 10,000) | 2 (1) | 20 (10) | ||
| Leukopenia (< 4,500) | 6 (3) | 49 (24.5) | ||
| Lymphocytes (n/mm3) | < 0.00001 | |||
| Normal | 40 (20) | 50 (25) | 35.4876 | |
| Lymphopenia (< 1,500) | 8 (4) | 102 (51) | ||
| Neutrophil (n/mm3) | Not significant | |||
| Normal | 47 (23.5) | 143 (71.5) | 0.4675 | |
| Neutrophilia | 1 (0.5) | 9 (4.5) | ||
| Platelets (n/mm3) | < 0.00001 | |||
| > 450000 | 8 (4) | 5 (2.5) | 31.3674 | |
| 150,000 - 450,000 | 30 (15) | 49 (24.5) | ||
| < 150000 | 10 (5) | 98 (49) | ||
| Hemoglobin (g/dL) | Not significant | |||
| Normal (> 12) | 43 (21.5) | 140 (70) | 0.0622 | |
| Hb < 12 | 5 (2.5) | 12 (6) | ||
| (mg/L) | Not significant | |||
| Normal | 2 (1) | 12 (6) | 0.3114 | |
| Abnormal | 46 (23) | 140 (70) | ||
| Troponin I (ng/L) | < 0.00001 | |||
| Normal | 6 (3) | 137 (68.5) | 104.1139 | |
| Abnormal | 42 (21) | 15 (7.5) | ||
| D-dimer (mic/L) | < 0.00001 | |||
| Normal | 28 (14) | 137 (68.5) | 23.3937 | |
| Abnormal | 20 (10) | 15 (7.5) | ||
| RT-PCR COVID-19 | 0.009752 | |||
| Positive | 8 (4) | 58 (29) | 6.6796 | |
| Negative | 40 (20) | 94 (47) |
Laboratory Findings in 200 Multisystem Inflammatory Syndrome in Children Patients in Two Groups of Cardiac Involvement and Normal Heart a
In this study, 48 (24%) patients with MIS-C had cardiac involvement, including ventricular dysfunction, coronary artery dilatation and aneurysm, mitral regurgitation, and pericardial effusion. The most common change in the electrocardiogram was sinus tachycardia in 39 (19.5%) patients. ST segment changes and negative T waves were seen in 11 (5.5%) cases, and sinus bradycardia was observed in 8 (4%) cases, with one of them having escape junction beats. One patient with severe left ventricular dysfunction developed ventricular tachycardia (VT) and fibrillation (VF) and unfortunately expired.
The echocardiogram showed 12 (6%) cases of MIS-C with mildly reduced EF, and 6 (3%) and 2 (1%) cases with moderately and severely reduced EF, respectively. Mitral regurgitation was seen in 16 (8%) cases. The rate of mitral regurgitation was mild in most cases. In 4 cases, the grade of mitral regurgitation was moderate. The grade of mitral regurgitation was significantly reduced after treatment with IVIG and corticosteroids. Mild pericardial effusion was detected in 20 (10%) cases, and in all of them, the pericardial effusion resolved gradually over time. Types of cardiac involvement in patients with cardiac complications are shown in Table 2.
| Echocardiogram/ Electrocardiogram Abnormalities | Values; No. (%) a |
|---|---|
| LVEF (%) | |
| > 55 (normal) | 28 (14) |
| < 55 | 20 (10) |
| EF 41 - 54 | 12 (6) |
| EF 31 - 40 | 6 (3) |
| EF ≤ 30 | 2 (1) |
| Coronary abnormalities | |
| Dilatation (Z score 2 - 2.5) | 6 (3) |
| Small aneurysm (Z score 2.5 - 5) | 2 (1) |
| Mitral regurgitation | 16 (8) |
| Pericardial effusion | |
| Yes | 22 (11) |
| No | 26 (13) |
| Sinus tachycardia | 39 (19.5) |
| Sinus bradycardia | 8 (4) |
| Ventricular tachyarrhythmia/VF | 1 (0.5) |
| Abnormal ST-T wave | 11 (5.5) |
| Cardiogenic shock | 8 (4) |
Types of Cardiac Involvement in 48 Cases Detected Through Echocardiography or Electrocardiography
Two cases (1%) developed small coronary aneurysms (Z score 2.5 - 5), and 6 (3%) patients showed transient coronary ectasias (Z score < 2.5). During treatment, there was no significant change in the size of the coronary artery. Ventricular dysfunction normalized in most patients within a median of 7 ± 2 days after treatment. Electrocardiographic abnormalities also resolved over time. Among the patients, 8 (4%) presented with cardiogenic shock and required inotropic therapy. In patients who had LV dysfunction, an increase in EF was observed in the one-year follow-up. In two patients who had an EF below 30%, after one year, EF reached 46% and 50%. In the one-year follow-up, small coronary aneurysms (Z score 2.5 - 5) remained in two patients. Pericardial effusion, mitral regurgitation, and electrocardiogram abnormalities disappeared in all patients at follow-up.
5. Discussion
The MIS-C is a hyperinflammatory state during COVID-19 infection with diagnostic criteria as pointed out in the introduction. The relationship between genetic susceptibility and triggering factors, which leads to hyperinflammatory responses and the development of MIS-C, is shown. Some reports indicate differences in the rate and severity of involvement in cases from different countries. This suggests the role of genetic susceptibility in the pathogenesis of MIS-C. Triggering factors are generally infectious agents, drugs, environmental agents, or traumas that cause immune-mediated responses and cytokine storm syndrome in children (18).
Although, according to the findings of some similar studies, the median age of confirmed cases in MIS-C is reported to be 7 - 11 years (19); in our study, it was lower, around 4 years. This difference may be due to racial differences. Our study showed an incidence rate of 1.74 percent for MIS-C among children with COVID-19 up to 12 years old. Some similar published studies have shown different results. The incidence of MIS-C in the study by La Torre et al. in Southern Italy was 3.27/100,000 (20), and in the cohort study of Luxembourg for ages 0 - 15 years old, it was 51/100,000 (21). Payne et al. found this incidence to be 31.6/100,000 in cases between 0 to 21 years old in the United States (22). These differences could be due to different age groups in cases and the potential genetic role in the occurrence of MIS-C.
Cardiac involvement in our cases was 24 percent of all MIS-C patients (48 of 200 cases), whereas previous studies revealed cardiac involvement in up to 67 - 80 percent of their patients (23), and Campanello et al. reported cardiac involvement in up to 84 percent of MIS-C cases (24). Kostik et al., following a multicenter cohort study, reported heart involvement in 41.9 percent of patients with MIS-C (25). It seems that cardiac involvement was less common in our cases compared to similar studies. On the other hand, based on the study by Wu and Campbell, decreased left ventricular function was observed in 34 - 50 percent of children with MIS-C (23), while it was only 10 percent in our cases, significantly less than their results. Pericardial effusion in the studies by Campanello et al. and Simon Junior et al. was detected in 80 and 48 percent of MIS-C cases with heart involvement, respectively (24, 26); whereas, it was just 11 percent in our patients.
In our study, the results indicated 8 individuals with coronary artery involvement (including 6 cases with dilatation and 2 with small aneurysms, totaling 4 percent of all MIS-C patients); other similar studies showed higher statistics. Campanello et al. reported 48% (24), Simon Junior et al. found 38% (25, 26), and Misra et al., through their study in 66 hospitals across 31 states in the USA, showed 20 percent coronary artery involvement (27). We detected 8 cases of cardiogenic shock among all the cases (16 percent). This was 19.8 percent in the report by Tahera Nazrin et al. from Bangladesh (28). Based on the report by Misra et al., the occurrence of hypotension was 52 percent in MIS-C cases (27). This report pointed to hypotension due to several causes, such as depressed left ventricular systolic function, persistent tachycardia, cardiogenic shock, and so on; therefore, we prefer not to compare.
Mitral regurgitation was observed in 16 cases (8 percent) in our study, and in studies by Campanello et al. and Tahera Nazrin et al. (28), it was 40 percent (24) and 13.32 percent (28), respectively. Comparison of our clinical findings showed some differences with similar studies; for instance, pericardial effusion and reduced LVEF were the two most common cardiac findings in this study, whereas in the study by Campanello et al., pericardial effusion and atrioventricular valve regurgitation were the two most common (24). Overall, compared to other similar studies, heart involvement was less common in our cases. Two reasons that could be considered are the role of genetic background and/or the probability of the effect of early initiation of IVIG and corticosteroid treatment for the patients. Proving each of these two probable causes requires further research. In one study of 55 patients with MIS-C, 38 percent ECG abnormalities were reported (29), while it was 29.5 percent in our study.
Laboratory findings in our study revealed important points: Comparing two groups — those with cardiac involvement and those with a normal heart — in all cases with MIS-C showed significant differences in some items, including white blood cell count (P = 0.001709), lymphocyte count (P < 0.00001), platelet count (P < 0.00001), the level of troponin I (P < 0.00001), and the level of D-dimer (P < 0.00001). Additionally, the results of RT-PCR for COVID-19 showed significance (P < 0.01). On the other hand, there was no significance between the two groups for neutrophil count, the level of hemoglobin, and CRP.
Based on our results, increased troponin I and D-dimer were more commonly seen in the condition of cardiac involvement; therefore, these inflammatory factors could be considered important markers. Similarly, thrombocytosis was found more in cases with heart involvement (P < 0.05). Thrombocytopenia was more likely seen in cases with a normal heart (64.5 percent) and was seen in only 20.8 percent of patients with cardiac involvement (P < 0.00001). Another significant finding in our study was the lower incidence of cardiac involvement in the presence of lymphopenia (lymphopenia in: 16.6 percent of cases with cardiac involvement vs. 67.1 percent in cases with a normal heart; P < 0.00001).
The rate of increase in troponin I in cases of cardiac involvement in our study was 87.5 percent, similar to the studies by Rodriguez-Gonzalez et al. and Jiang et al., which were 86.8 percent and 80.9 percent (30, 31), respectively. Contrary to these results, some studies showed lower rates of increased troponin I, such as the studies by Kochi et al. with 16.7 percent (32) and Dionne et al. with only 8 percent (33). Among our patients, individuals with reduced LVEF and pericardial effusion had higher levels of inflammatory markers than others.
LV dysfunction was present in 10% of the patients at the beginning of this study, with full recovery observed in all except two patients. Coronary involvement was observed in 8 patients (4%) of the children during the acute phase. In the one-year follow-up, small coronary aneurysms (Z score 2.5 - 5) remained in two patients. Pericardial effusion, mitral regurgitation, and electrocardiogram abnormalities disappeared in all patients at follow-up. The results of our follow-up in this study are almost similar to the study by Cantarutti in Italy (34). Studies about the follow-up of children with cardiac involvement in MIS-C are very limited. Longer-term studies are required to understand the clinical course of cardiac involvement in children with MIS-C.
5.1. Clinical Implications and Management Considerations
The findings of our study provide valuable insights for clinicians managing MIS-C, particularly in populations with lower rates of cardiac involvement. Several key points emerge from our data:
1. Early diagnosis and risk stratification: (1) Elevated levels of troponin I, D-dimer, and thrombocytosis were strongly associated with cardiac involvement in our study, making them useful biomarkers for identifying high-risk patients; (2) Conversely, lymphopenia was more prevalent in patients without cardiac involvement, suggesting that its presence may indicate a different inflammatory trajectory.
2. Role of early IVIG and corticosteroid therapy: (1) Our data suggest that early administration of IVIG and corticosteroids may contribute to a lower incidence of severe cardiac complications; (2) This aligns with emerging evidence supporting the use of early anti-inflammatory treatment to reduce cardiovascular involvement in MIS-C.
3. Prognosis and long-term monitoring: (1) Most patients with reduced LVEF or pericardial effusion recovered fully, except for two cases with persistent small coronary aneurysms; (2) One-year follow-up demonstrated a good overall prognosis, with resolution of mitral regurgitation and ECG abnormalities in all cases; (3) These findings underscore the importance of structured follow-up to monitor for late cardiac complications, even in patients who initially present with mild or moderate disease.
4. Comparisons with global data and future directions: (1) The lower rates of cardiac involvement in our study compared to Western cohorts highlight the need for population-specific guidelines in MIS-C management; (2) Future studies should focus on genetic and immunological factors that may contribute to variations in disease severity across different ethnic and racial groups; (3) Long-term follow-up studies are essential to determine whether early treatment strategies provide sustained cardiac protection beyond the one-year mark.
5.2. Conclusions
In this study, we found less common cardiac involvement compared to similar studies, and this lower rate was observed in all components, including decreased LVEF, coronary abnormalities, pericardial effusion, valvular disorders, ECG abnormalities, and cardiogenic shock. This may be due to differences in genetic background or related to the early use of IVIG and corticosteroids. Cardiac involvement in the one-year follow-up showed a good prognosis. Some laboratory findings showed significance between the two groups of cardiac involvement and normal heart among MIS-C patients, including WBC count, lymphocyte count, platelet count, and the levels of troponin I and D-dimer; for all these items, P was less than 0.05. On the other hand, no significant difference was seen for neutrophil count and the levels of hemoglobin and CRP. Thrombocytosis was significantly more common in cases of cardiac involvement (P < 0.05), whereas in cases with a normal heart, the occurrence of lymphopenia and thrombocytopenia was significantly higher (for both items: P < 0.05). Furthermore, elevated troponin I, D-dimer, and thrombocytosis emerged as potential biomarkers for predicting cardiac involvement.
5.3. Limitations
This study has a few important limitations. First, it was conducted at a single referral center, which may limit the generalizability of the findings to other populations or regions. Second, we relied mainly on echocardiography and ECG to assess cardiac involvement. More detailed imaging, such as cardiac MRI, was not available at our center; therefore, subtle heart issues could be missed. Third, although all patients were followed for one year, we cannot draw conclusions about longer-term cardiac outcomes beyond that period. Fourth, while we suggested that genetic background or early treatment might explain the relatively mild cardiac involvement in our patients compared to similar studies, we did not include any genetic testing or multi-center comparisons to explore this further. Fifth, we did not adjust for potential confounding factors (such as underlying comorbidities, socioeconomic status, or timing of treatment), which may have influenced the observed cardiac outcomes. In addition, the absence of a predetermined sample size may have reduced the statistical power to detect subtle differences in less prevalent outcomes, potentially limiting the ability to generalize findings to other settings with different MIS-C prevalence rates. Future multi-center, prospective studies with control groups and more advanced imaging tools are needed to validate and expand on these observations.
5.4. Future Research Directions
While our study provides valuable insights, several key questions remain unanswered. Future research should focus on the following areas to better understand the role of genetics and treatment timing in MIS-C-related cardiac involvement:
1. Genetic and immunological studies: Large-scale genome-wide association studies (GWAS) could help identify genetic markers associated with lower cardiac involvement in MIS-C.
2. Treatment timing and effectiveness: Prospective cohort studies comparing early versus late administration of IVIG and corticosteroids could determine how timing affects cardiac outcomes.
3. Long-term cardiac follow-up: Multicenter longitudinal studies tracking MIS-C patients over several years would help determine whether early treatment leads to lasting cardiac protection.
4. Advanced imaging studies: Advanced imaging studies, such as cardiac MRI, could provide more detailed assessments of long-term myocardial function and coronary artery health.