1. Background
Breast cancer is one of the most common cancers among women, less frequent among men, and the second leading cause of death in women (1). Breast cancer is highly heterogeneous and consists of several subtypes (2). Based on the immunohistochemical expression of hormone receptors, these subtypes are typically categorized into four groups: Estrogen receptor-positive (ER+), progesterone receptor-positive (PR+), human epidermal growth factor receptor-positive (HER2+), and triple-negative breast cancer (TNBC), which is defined by the absence of expression of the mentioned receptors. Studies have demonstrated that cytokines play a crucial role in cancers and can act as stimulators or inhibitors of cancer development (3).
One of the most significant cytokines in the immune system is tumor necrosis factor (TNF-α), a member of the TNF superfamily (4). TNF-α is involved in various pathological conditions, including rheumatoid arthritis (5), osteoarthritis (6), and inflammatory bowel disease (7). In cancer pathogenesis, TNF-α regulates pathways related to cell proliferation, differentiation, survival, and death (8). Tumor necrosis factor-α functions as a double-edged sword in cancer biology. While it contributes to the initiation of neoplastic transformation and promotes DNA instability, it can also act as an autocrine growth factor for tumor cells, thereby supporting tumor progression and metastasis. Furthermore, TNF-α affects nearly all cell types in the human body through complex signaling pathways such as MAPK, Akt, and NF-κB. This widespread influence complicates its potential use as an anti-cancer therapy, as its involvement in both pro-survival and anti-survival mechanisms poses significant therapeutic challenges (9).
Regarding breast cancer, TNF-α has been shown to play a role in linking inflammation with breast cancer growth (10). Moreover, individuals with elevated TNF-α concentrations face a higher risk of developing breast cancer.
2. Objectives
In this study, we aimed to investigate the serum levels of TNF-α in different subtypes of breast cancer in Iranian women.
3. Methods
In this study, 114 serum samples from patients with different types of breast cancer were collected. The study was approved by the Ethics Committee of Qazvin University of Medical Sciences (IR.QUMS.REC.1401.343), and informed consent was obtained from each patient. The study protocol adhered to the principles of the Declaration of Helsinki. The pathological data, including the types of breast cancer and different stages, for each participant were obtained from the patients' medical records and confirmed by an experienced histopathologist (Table 1). The serum concentration of TNF-α was measured using the ELISA technique with the human TNF-α ELISA kit (Thermo Fisher, USA) following the manufacturer’s protocol.
| Types of Samples | N | Age | Numbers |
|---|---|---|---|
| Her-2 positive | 31 - 61 | 34 | |
| I | 15 | ||
| II | 10 | ||
| III | 9 | ||
| ER-positive | 33 - 59 | 27 | |
| I | 7 | ||
| II | 12 | ||
| III | 8 | ||
| PR-positive | 34 - 61 | 33 | |
| I | 15 | ||
| II | 10 | ||
| III | 8 | ||
| Triple negative | 31 - 48 | 20 | |
| I | 2 | ||
| II | 8 | ||
| III | 10 |
4. Results
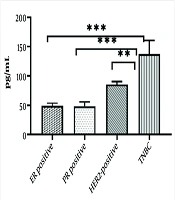
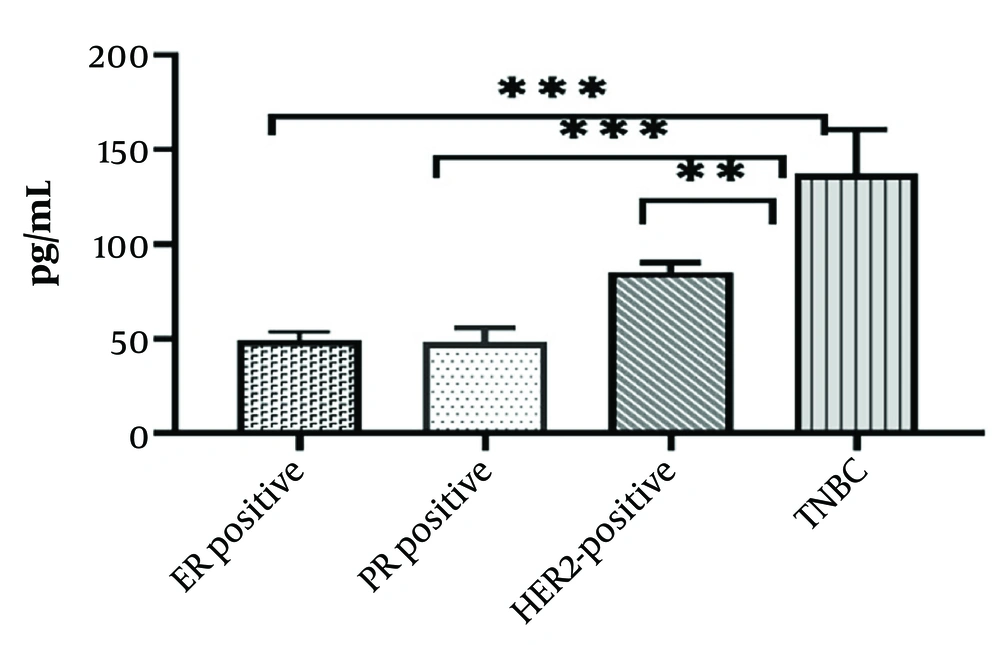
Based on these results, there was a significantly higher concentration of TNF-α in the serum samples of patients with TNBC compared to other subtypes (P < 0.0001) (Figure 1).
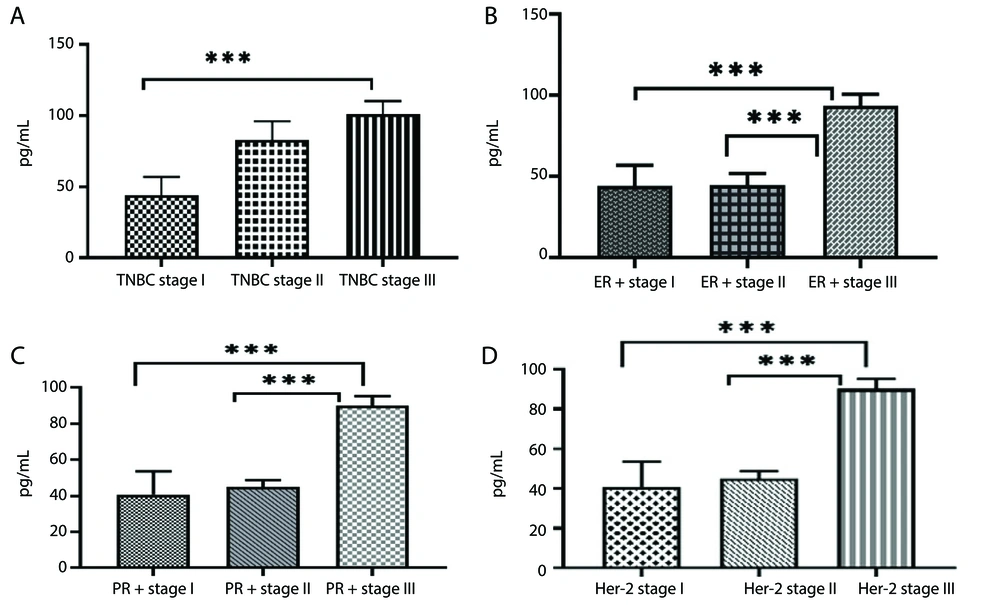
In another part of the study, we compared the concentration of TNF-α across different stages of cancer within each subtype. The results showed a higher concentration of TNF-α in stage III compared to stages I and II within each subtype (P < 0.0001) (Figures 2A - D).
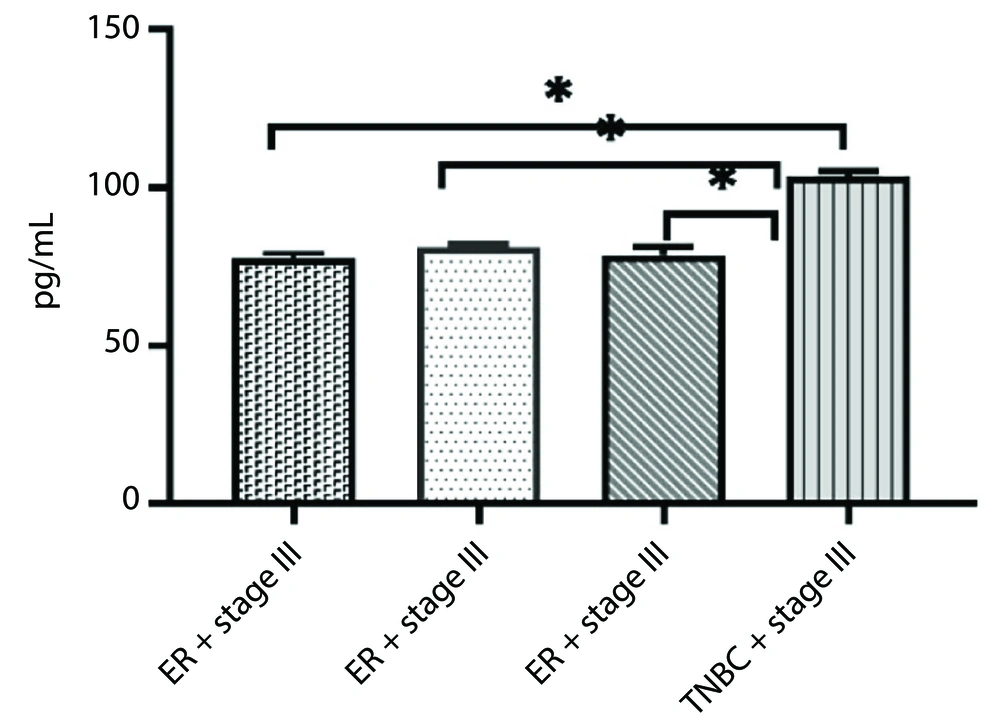
Additionally, there was a significant difference in tumor necrosis factor-α (TNF-α) levels between stage III TNBC patients and those in the other groups (P < 0.05) (Figure 3).
5. Discussion
In this study, we reported a high concentration of TNF-α in stage III TNBC compared to ER-, PR-, and HER2-positive samples. The lack of clinically significant ER, PR, and HER2 receptor levels is a characteristic of TNBC. This particular type of breast cancer represents 15 - 20% of all breast cancer cases and is more common in younger women (< 35 years) (11). It has a 77% survival rate, is typically diagnosed at an advanced stage, carries a significant risk of metastasis, and demonstrates a poor response to therapy (11).
The results of different studies regarding the role of TNF-α in TNBC are contradictory. Qiao et al. showed that TNF-α causes apoptosis in BT549 cells, downregulates anti-apoptotic genes, and increases the expression of pro-apoptotic genes. In contrast, Qiao et al. also reported that TNF-α has a malignant effect (12). Another study demonstrated that TNF-α did not trigger apoptosis in TNBC cell lines (13). Some reports have shown that TNF-α is associated with cancer cell motility, invasion, and epithelial-mesenchymal transition (EMT) (14, 15). Consistent with these studies, we reported a high concentration of TNF-α in patients with stage III breast cancer.
An important protein involved in malignancy induced by TNF-α is MMP-9, which is activated by TNFα-induced AP-1 activation (16) or CDKNA1/p21 (17), and can act as an oncogene (18). Another contributing factor is the A20 protein, which stimulates the inflammatory IL-6/phospho-STAT3 pathway. This pathway upregulates HSP70 and inflammatory cytokine production, including IL-6, IL-8, CCL5, and TGF-β, upon TNFα stimulation. In TNBC cell lines, this results in an aggressive phenotype, increased metastatic potential, and the production of cancer stem cells (19, 20).
5.1. Conclusions
In conclusion, our results demonstrated that TNF-α plays a significant role in the malignancy and metastasis of breast cancer, particularly in TNBC. These findings suggest that drugs targeting the inhibition of TNF-α may hold potential as effective treatments for patients with this aggressive subtype of breast cancer.