1. Background
Coronavirus disease 2019 (COVID-19) is a viral disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1). Coronavirus infection was declared a pandemic in 2020 (2). The disease causes a spectrum of infections, ranging from mild respiratory symptoms to severe respiratory disorders, such as organ failure (3) and acute respiratory distress syndrome (ARDS), which can lead to death (4).
Moreover, during the SARS-CoV-2 pandemic, the combination of respiratory failure and metabolic changes resulting from respiratory and renal dysfunction has led to unpredictable electrolyte and acid-base imbalances (5). Similar to SARS-CoV, SARS-CoV-2 utilizes the ACE2 receptor to exert its pathogenic effects. The virus enters cells by binding to ACE2 receptors (6, 7). Due to the high levels of ACE2 receptors in kidney cells, specifically podocytes and tubule epithelial cells, the kidneys are particularly susceptible to coronavirus invasion. As a result, acute renal impairment due to COVID-19 is not unexpected. One of the kidney's critical roles is maintaining the acid-base balance; however, its dysfunction may exacerbate COVID-19 outcomes (8).
Acid-base imbalances arising from renal impairment can result in metabolic acidosis, typically caused by the accumulation of acids or the loss of bicarbonate from the gastrointestinal tract (9). Conversely, metabolic alkalosis may develop from the accumulation of bases or acid loss (10).
In addition to acid-base imbalances originating from the kidney, respiratory types can also occur. Respiratory acidosis arises due to impaired ventilation, leading to carbon dioxide accumulation, further complicating the clinical course of COVID-19. Additionally, respiratory alkalosis may develop due to acute or chronic processes, such as hyperventilation (11), which can be triggered by hypoxemic causes and pulmonary illnesses. Respiratory alkalosis is one of the most commonly observed acid-base imbalances in clinical practice and was identified in the arterial blood of intensive care unit (ICU) patients in our study (12). However, the relationship between the incidence of respiratory acid-base disorders and COVID-19 progression remains unclear in some COVID-19 patients presenting with dyspnea (11).
Given the critical importance of these acid-base imbalances, frequent arterial blood gas (ABG) analyses are necessary to monitor changes in acid-base balance and disease progression (12). On the other hand, several studies have clarified that electrolyte imbalances in admitted COVID-19 patients could serve as risk indicators for severe illness and mortality (13-16). Electrolyte status in COVID-19 patients can significantly impact treatment outcomes, hospital stay duration, and mortality (12).
The incidence and outcomes of acid-base disorders in COVID-19 patients remain insufficiently assessed (17). Previous studies have demonstrated that angiotensin II can enhance aldosterone secretion, leading to renal sodium (Na) reabsorption and potassium (K) excretion. This highlights the potential role of the ACE2 receptor in contributing to electrolyte abnormalities, particularly those involving Na and K (18). Various studies have identified acid-base disorders as significant prognostic factors for the severity of diseases in critically ill patients, particularly those admitted to ICU-CCU settings.
2. Objectives
Considering their potential impact on clinical outcomes, we aimed to study the prevalence of pH and electrolyte disorders in ICU-admitted patients and their effects on mortality and the need for mechanical ventilation (intubation).
3. Methods
3.1. Study Design and Patients
This retrospective study was conducted from 2020 to 2022 on 250 patients with laboratory-confirmed COVID-19 who were admitted to the ICU of Velayat Hospital, Qazvin, Iran. The inclusion criteria included patients over 16 years of age, hospitalized in the ICU for at least 24 hours, and confirmed positive for SARS-CoV-2 by RT-PCR. Patients were considered to have severe illness if they had an SO2 < 94% on room air at sea level (5) and pulmonary involvement of more than 50%, as indicated by chest CT scan reports. Additionally, the NIH criteria for severe SARS-CoV-2 were applied in this study. Patients taking diuretics, glucocorticoids, or corticosteroid drugs were excluded from the study. Those with a previous history of moderate or severe kidney or liver failure were also excluded. Informed consent was obtained from all patients before their inclusion in the study. This study was approved by the local Medical Ethics Committee of the Qazvin University of Medical Sciences (IR.QUMS.REC.1400.394).
3.2. Clinical Assessments and Data Collection
Descriptive data, including demographic information on age, sex, underlying disease (diabetes, hypertension, and diseased cardiac ischemia), height and weight Body Mass Index (BMI), average length of hospital stay, mechanical ventilation, and its duration, were retrieved. Laboratory test reports comprising erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), blood urea nitrogen (BUN), creatinine (Cr), aspartate transaminase (AST), alanine transaminase (ALT), ALK, lactate dehydrogenase (LDH), Na, K, calcium (Ca), phosphate (P), magnesium (Mg), albumin (Alb), fasting blood sugar (FBS), white blood cell (WBC) count, lymphocyte count, polymorphonuclear (PMN) count, hemoglobin (Hb), hematocrit (HCT), anion gap (AG), chloride (Cl), LFT, VBG, and ABG (pH, PCO2, PO2, HCO3, SO2) were gathered, as well. All laboratory parameters were examined in all COVID-19 patients on the first day of hospitalization in the ICU. Also, VBG and ABG tests were performed on patients admitted to the ICU to assess the gas and pH levels. Therefore, regarding the relationship between COVID-19 prognosis and acid-base imbalances, the conditions of pH > 7.45, PCO2 < 35 mmHg, and pH < 7.35, PaCO > 45 mmHg in ABG are considered respiratory alkalosis and respiratory acidosis, respectively (11).
The amount of AG in these patients can be calculated by measuring blood Cl, Na, K, and HCO3, which helps to distinguish possible acid-base disorders (19). Hence, the parameters mentioned above were retrieved from patients to detect AG abnormalities, as well as other diseases. Also, information about the delivered serum, the degree of oxygen saturation of arterial blood with or without oxygen administration, determination of the amount of delivered oxygen, method of oxygen delivery (e.g., nasal cannula, simple oxygen mask, etc.), blood pressure, and respiratory rate of patients were noted. Eventually, patients were classified into ward (patients moved to the ward after ICU under the criteria of no dependence on mechanical ventilation, O2 saturation of more than 94%, stable blood pressure, and good health status), ICU (patients who remained in the ICU), and expired (patients who died in the ICU) groups based on the patients’ medical records.
3.3. Statistical Analysis
All statistical analyses were performed using SPSS 23 software. Descriptive results are presented as frequencies, means, and standard deviations based on the variable type. Chi-squared and independent t-test were used to examine the relationships between qualitative and quantitative variables, respectively. The Pearson correlation coefficient also analyzed the data to investigate the relationship between quantitative variables and acid-base disorders or their nonparametric equivalents. A significance level of less than 0.05 was considered. Logistic regression analysis was also applied in order to investigate the effects of clinical variables on the clinical status and intubation status outcomes. The ANOVA test evaluated the relationship between categorical independent and quantitative dependent variables (paraclinical parameters).
4. Results
4.1. Patients’ Demographic and Clinical Data
This study was conducted on 250 COVID-19 patients, of which 69 (27.6%) were admitted to the ICU, 140 (56%) were admitted to the hospital ward, 41 (16.4%) died, and 49 (19.6%) underwent mechanical ventilation. Of all patients, 114 (45.6%) were men, and 136 (54.4%) were women. The estimated mean age of the population was 58.16 ± 17.86, with a female predominance. According to the underlying disease analysis, diabetes was the most frequent underlying disease. Seventy seven (73%) patients had diabetes and 28 (27%) had ischemic heart disease. The paraclinical parameters were also measured, as shown in Table 1.
| Paraclinical Parameters | Mean ± SD |
|---|---|
| Age (y) | 58.16 ± 17.86 |
| BMI (kg/m2) | 27.84 ± 4.70 |
| pH | 7.39 ± 0.08 |
| PCO2 (mmHg) | 36.74 ± 8.76 |
| PO2 (mmHg) | 48.05 ± 23.69 |
| HCO3 (mq/L) | 22.64 ± 4.39 |
| Na (mEq/L) | 136.91 ± 3.44 |
| K (mEq/L) | 4.11 ± 0.50 |
| Ca (mg/dL) | 8.39 ± 0.65 |
| P (mg/dL) | 4.18 ± 1.61 |
| Alb (g/dL) | 3.45 ± 0.53 |
| Mg (mg/dL) | 2.15 ± 0.39 |
| WBC count (× 109/L) | 10.16 ± 8.07 |
| Lymph count (%) | 12.41 ± 10.42 |
| PMN count (%) | 82.49 ± 9.52 |
| Hb (g/dL) | 12.41 ± 2.16 |
| HCT (%) | 37.18 ± 6.21 |
| LDH (IU/L) | 818.7 ± 384.64 |
| CRP (mg/L) | 58.94 ± 29.37 |
| ESR (mm/h) | 58.24 ± 30.04 |
| FBS (mg/dL) | 171.7 ± 75.13 |
| BUN (mg/dL) | 23.58 ± 18.65 |
| Cr (mg/dL) | 1.32 ± 1.18 |
| AST (IU/L) | 63.91 ± 65.93 |
| ALT (IU/L) | 55.69 ± 52.15 |
| ALP (IU/L) | 250.35 ± 238.38 |
| AG (mEq/L) | 6.49 ± 4.61 |
| SO2 (%) | 90.25 ± 6.92 |
| RR (breaths/min) | 23.02 ± 5.58 |
| BPs (mmHg) | 122.82 ± 20.54 |
| BPd (mmHg) | 73.8 ± 14.34 |
| Cl (mEq/L) | 109.31 ± 5.59 |
Demographic and Paraclinical Parameters
Paraclinical parameters were also analyzed according to status (ICU-admitted, ward-admitted, and expired patients) and mechanical ventilation classification. Significant differences between the Po2, age, BUN, SO2, LDH, BPd, and the status were observed. Moreover, statistically significant differences between mechanical ventilation and age, pH, phosphorus, Mg, lymphocyte count, PMN count, LDH, and O2 saturation were revealed. It is worth mentioning that Na could be considered an essential factor affecting the need for mechanical ventilation (P-value = 0.05) (Appendix 1 in Supplementary File).
4.2. Classification of Patients
Based on these three parameters, the patients were divided into three classifications as follows: (1) According to pH: Respiratory acidosis, respiratory alkalosis, metabolic acidosis, and metabolic alkalosis; (2) according to K levels: (A) Mild hypokalemia (3.5 - 4.5 mEq/L); (B) moderate hypokalemia (3 - 3.5 mEq/L); (C) severe hypokalemia (< 3 mEq/L); (D) hyperkalemia (4.5 - 6 mEq/L); (3) according to Na levels: (A) Mild hyponatremia (130 - 135 mEq/L); (B) moderate hyponatremia (120 - 130 mEq/L); (C) severe hyponatremia (< 120 mEq/L); (D) normal Na (135 - 145 mEq/L).
4.2.1. pH Disturbances
Considering the ABG analyses, 17 (6.8%) patients, with a mean age of 56.53 years, had respiratory acidosis. Metabolic acidosis was observed in 47 (18.8%) patients, with a mean age of 60.11 years. Additionally, 126 (50.4%) patients, with a mean age of 57.8 years, had respiratory alkalosis, while 60 (24.0%) patients, with a mean age of 57.85 years, experienced metabolic alkalosis. Based on pH disturbances, the majority of ICU-admitted, expired, and ward-admitted patients were in the respiratory alkalosis group. Furthermore, most intubated (mechanically ventilated) patients also suffered from respiratory alkalosis (Table 2). However, no significant differences were found between pH disturbances, mechanical ventilation, and patient status.
4.2.2. Potassium Levels
According to K levels, hypokalemia was observed in a large group of 187 (74.8%) patients, while 15 (6%) patients had moderate hypokalemia, and 1 (0.4%) patient experienced severe hypokalemia. Potassium levels were elevated to the level of hyperkalemia in 47 (18.8%) patients. Based on K levels, the majority of ICU-admitted, expired, and ward-admitted patients were in the mild hypokalemia group. Similarly, most intubated (mechanically ventilated) patients also had mild hypokalemia (Table 3). There was no significant difference between K levels and mechanical ventilation. Additionally, no significant difference was found between K classification and the patients’ status. However, K levels differed significantly based on the patients’ status (P = 0.002).
4.2.3. Sodium Levels
Based on Na levels, 87 (34.8%) patients were identified as having mild hyponatremia, 5 (2%) patients had moderate hyponatremia, and the majority of patients (63.2%) had Na levels within the normal range. The majority of ICU-admitted, expired, and ward-admitted patients fell into the normal Na group, with no significant difference observed between status and Na classification. On the other hand, most intubated (mechanically ventilated) individuals had mild hyponatremia (Table 4). Additionally, there was a close relationship between Na levels and mechanical ventilation (P = 0.05).
4.3. Pearson Correlation Analyzes
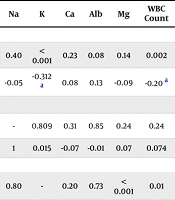
Pearson correlation was utilized to assess the significance of the relationships between the pH, Na, and K levels and the paraclinical parameters. In this way, correlation results are listed in the table below (Table 5).
| Variables | pH | Na | K | Ca | Alb | Mg | WBC Count | Lymph Count | PMN Count | Hb | HCT | LDH | CRP | ESR | FBS | BUN | Cr | AST | ALT | AG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | ||||||||||||||||||||
| P | - | 0.40 | < 0.001 | 0.23 | 0.08 | 0.14 | 0.002 | 0.05 | 0.17 | 0.03 | 0.09 | 0.29 | 0.13 | 0.60 | 0.69 | < 0.001 | < 0.001 | 0.93 | 0.29 | 0.003 |
| r | 1 | -0.05 | -0.312 a | 0.08 | 0.13 | -0.09 | -0.20 a | 0.12 | -0.09 | 0.13 b | 0.10 | -0.07 | 0.09 | 0.03 | -0.02 | -0.31 a | -0.37 a | -0.005 | 0.06 | -0.18 a |
| Na | ||||||||||||||||||||
| P | 0.40 | - | 0.809 | 0.31 | 0.85 | 0.24 | 0.24 | 0.65 | 0.48 | 0.92 | 0.35 | 0.58 | 0.32 | 0.42 | 0.73 | 0.05 | 0.54 | 0.19 | 0.64 | < 0.001 |
| r | -0.05 | 1 | 0.015 | -0.07 | -0.01 | 0.07 | 0.074 | -0.03 | 0.047 | 0.006 | 0.05 | 0.03 | -0.06 | -0.05 | -0.02 | 0.12 | -0.03 | 0.08 | -0.03 | 0.31 a |
| K | ||||||||||||||||||||
| P | < 0.001 | 0.80 | - | 0.20 | 0.73 | < 0.001 | 0.01 | 0.08 | 0.11 | 0.23 | 0.04 | 0.00 | 0.04 | 0.46 | < 0.001 | < 0.001 | < 0.001 | 0.008 | < 0.001 | 0.006 |
| r | -0.31 a | 0.01 | 1 | 0.08 | -0.02 | 0.19 a | 0.15 b | -0.11 | 0.10 | 0.07 | 0.12 a | 0.17 a | -0.13 b | -0.04 | 0.20 a | 0.33 a | 0.24 a | 0.17 a | 0.19 a | 0.17 a |
Paraclinical Parameters Correlation
4.4. Logistic Regression Analysis
Logistic regression analysis was conducted to evaluate the effect of clinical variables on clinical status, considering age, K, Po2, PMN count, LDH, BUN, and SO2. The analysis revealed that an increase in age of patients was associated with an adverse status in COVID-19 patients. Specifically, the regression analysis indicated that with every year of age, the chance of death increased by 4% (P = 0.01, OR = 1.04). Conversely, SO2 showed an inverse relationship with the risk of death. An increase in SO2 by 1 unit was associated with an 8% reduction in the odds of death (P = 0.007, OR = 0.92). Additionally, logistic regression was applied to assess the relationship of Mg, age, pH, P, lymph count, PMN, LDH, BUN, SO2, and Na with the need for mechanical ventilation. Following the analysis, only Na remained in the regression model. The results demonstrated that an increase in Na by 1 unit reduced the risk of mechanical ventilation by 41% (P < 0.001, OR = 0.59), highlighting its critical role in predicting mechanical ventilation requirements.
5. Discussion
Coronavirus disease 2019 disease, one of the most important diseases of the current century, can progress from mild respiratory infections to severe respiratory disorders, including organ failure, and may finally lead to death (20). Therefore, comprehensive monitoring of COVID-19 patients is vital for optimal management of the patient’s status and for predicting disease progression.
In this regard, monitoring underlying diseases such as syndrome of inappropriate antidiuretic hormone secretion (SIADH)–which may exacerbate electrolyte/acid-base disturbances–can aid in the early diagnosis of high-risk patients who are highly likely to manifest adverse signs of COVID-19 (21, 22). Thus, we obtained related data.
Similar to a study by Patel et al., most COVID-19 patients with underlying diseases suffered from diabetes (23). Additionally, the potential influence of certain factors, including iatrogenic causes, endocrinopathies, and inadequate renal/respiratory compensation of acid-base imbalances, was excluded from the present study.
Putting underlying diseases aside, ACE2, as an important receptor of SARS-CoV-2, plays a key role in human pathogenicity (8). This receptor generally participates in the regulation of electrolyte homeostasis, cardiovascular health, and blood pressure balance (24), particularly in cases of extracellular fluid volume imbalance (18). Due to the virus's affinity for the lungs and kidneys, where ACE2 expression is highest, many acid-base and electrolyte disturbances may arise, stemming from pneumonia and kidney damage, respectively (25). Acid-base alterations within an organism (19) can lead to critical multi-organ effects (26).
Given the high concentration of active ACE2 in lung alveoli (27), pneumonia is the most common symptom of COVID-19 and is diagnosed in all hospitalized COVID-19 patients. Extensive pneumonia, a severe infectious condition, disrupts respiratory gas exchange, and as a result, acid-base disturbances originating from the respiratory system are expected in COVID-19 patients (17). Recent studies indicate that hyperventilation can occur following hypoxic conditions as the body attempts to correct hypoxia, leading to CO2 loss (28). This phenomenon suggests that respiratory alkalosis may result from hypoxia-induced hyperventilation, while respiratory acidosis can develop in patients with hypercapnic respiratory failure (17). Consequently, acid-base monitoring is crucial during the first day of admission to address potential clinical concerns and to provide timely interventions (20). A study by Bezuidenhout et al. reported, for the first time, that most ICU-admitted COVID-19 patients experienced alkalosis (12). Similarly, Wu et al. analyzed the clinical properties of patients with and without respiratory alkalosis, finding that the frequency of respiratory alkalosis on admission was approximately 28.7%, and it was associated with a higher risk of severe symptoms (11). In another study, Chiumello et al. found that up to 40% of COVID-19 patients with ARDS had respiratory alkalosis, while 32% had metabolic alkalosis (20). These findings were consistent with a study by Alfano et al., which observed respiratory alkalosis and metabolic alkalosis frequencies of 31% and 33%, respectively, in COVID-19 patients admitted within the first 48 hours (17). Respiratory and metabolic alkalosis can serve as prognostic factors for the progression of COVID-19 to ARDS. Consistent with prior studies, our study demonstrated that respiratory alkalosis was present in 51.4% of ward-hospitalized patients, 55.1% of ICU-admitted patients, and 39% of expired patients. Most of our patients experienced respiratory alkalosis, similar to the trends reported in other studies. Interestingly, the most frequent acid-base disturbance in expired patients was respiratory alkalosis, although the difference was statistically insignificant.
Metabolic alkalosis was observed in 20.7% of ward-hospitalized patients, 27.5% of ICU-admitted patients, and 29.3% of expired patients. As noted earlier, kidney impairment due to the virus’s renal affinity may contribute to metabolic alkalosis (25). Potential mechanisms of renal impairment leading to metabolic alkalosis in COVID-19 patients include: Fluid imbalance caused by fever-induced dehydration, loss of appetite, or reduced fluid intake (29). Coronavirus disease 2019 may have carried out its role in metabolic alkalosis by upregulation of the classic pathway of RAS. It may have taken part in increasing the aldosterone level, leading to hydrogen ion secretion from renal tubular cells that may cause metabolic alkalemia. On top of that, excessive mineralocorticoid activation could be another possible activator of metabolic alkalosis. Alternatively, according to literature, the use of corticosteroids has been found to contribute to metabolic alkalosis because of their effect on the mineralocorticoid system (12). Based on the impacts of SARS-COV-2 on the kidney and the development of metabolic alkalemia, our study also confirmed that pH levels were significantly correlated with BUN and CR levels. Consequently, the pH alteration may have affected renal function. Along with our findings, Longo et al. showed a significant pH level enhancement correlating with the BUN raise. Kidney impairment followed by pH increment during the kidney injury caused trouble extracting excess protons (raised by injured muscles) from blood to the ultrafiltration (30). In addition, we observed a significant correlation between pH level and WBC count as well as a significant correlation between pH level and Hb using Pearson correlation. The exact mechanism of these incidents has not been revealed until now. However, we believe that the multiorgan involvement by SARS-COV-2 affected inflammation development correlating with WBC count elevation.
Besides the importance of acid-base disturbances, we noticed that serum electrolyte abnormalities as one of the manifestations of acid-base balance disorder (31) could be relevant to COVID-19 progression toward severe forms like ARDS. In this way, it is reported that hypokalemia has been one of the most frequent electrolyte disorders in COVID-19 hospitalized patients (32). Although increased K levels correlate with various arrhythmias regardless of COVID-19 status, ventricular arrhythmia (33) and bradyarrhythmia occur possibly following K changes during COVID-19 disease (34). On the other hand, the most important predisposing risk factor for hypokalemia is respiratory alkalosis due to hypoxia-driven hyperventilation, which may stimulate transcellular shifts with elevated intracellular uptake (32). Aldosterone and RAAS activation may cause enhanced K secretion resulting in hypokalemia (35). Cytopathic effect of the virus on the gastrointestinal cells and medication (e.g., lopinavir, ritonavir) are other possible candidates leading to hypokalemia through anorexia and diarrhea (32). Sufficient plasma K levels play a notable role in preventing myocardial failure (36). severe acute respiratory syndrome coronavirus 2 can result in heart dysfunction due to the extensively presented ACE2 on the patients’ myocardial cells. Therefore, it is vital to monitor plasma K levels in patients admitted to the hospital due to its noteworthy role in predicting myocardial failure. This myocardial failure can result from the invasion of SARS-CoV-2 to myocardial cells expressing high levels of ACE2 (36). In our study, we observed that mild hypokalemia and respiratory alkalosis were the most frequent symptoms in our patients. Nasomsong et al. similarly reported that most COVID-19 patients had (94.4%) mild hypokalemia in the hypokalemia group (37). Moreover, we showed that K levels had a significant association with the mortality status of patients, which confirms the central role of K as one of the predictors of patients’ outcomes. Liu et al. reported that patients with COVID-19 having K levels ≥ 5.0 mmol/L had a significantly increased risk of death (38). Interestingly, in our study, there was a significant relationship between the pH level and the K level and K classification, which highlights the effect of acid-base status on hypokalemia. Also, in a study by Chen et al., a pH level higher than 7.45 (28%) was observed in patients with severe hypokalemia. One of the reasons may have likely been the severe hypokalemia which led to alkalosis due to H+ - K+ exchange between intracellular and extracellular fluid (36). A cellular K uptake was also observed by Aronson and Giebisch in the alkalosis condition (39). Based on the studies previously mentioned, pH changes, especially respiratory alkalosis, can generate hypokalemia leading to death and a severe condition in COVID-19 patients.
In addition, there were several paraclinical parameters investigated in our study. In this way, we found a significant relationship between K classification and age, similar to a study conducted on COVID-19 patients by Anderson and Langham, in which they reported that age-adjusted muscle mass was related to serum K concentration (40). There was also a statistically significant association between FBS and K classification. Likewise, a study accomplished by Chatterjee et al. showed that experimentally-induced hypokalemia caused a reduction in insulin secretion, which indicates K’s possible role in diabetes mellitus (41). In our study, a significant relationship was observed between K and serum CR levels, similar to a study by Takaichi et al., indicating that a serum K increment significantly correlated with increased serum CR and impaired renal function, possibly leading to hyperkalemia (42).
In addition, some other paraclinical tests having a significant relationship with K classification were obtained, counting BUN, HCO3, ALP, ALT, HCT, WBC count, Mg, and P. Also, significant correlations between the CRP, AST, LDH, and K levels were observed.
In the present study, other analyses were accomplished based on the Na classification and Na levels. Previous studies showed Na levels had high predictive power for COVID-19 disease progression (4). Since the ACE2 receptor is highly present in the proximal tubule, hyponatremia is one of the manifestations of COVID-19 (43). On the other hand, angiotensin II can release aldosterone into the blood, causing Na reabsorption from the nephron and increasing the excretion of H+ ions into the urine (44). Moreover, the RAS disturbance can also increase the reabsorption of Na (36). Related studies conducted in this field have controversial results, so most studies declared that hyponatremia significantly correlated with patients’ mortality and severe manifestation (45, 46). Also, a few studies mentioned hypernatremia as a correlating factor with COVID-19 patients’ mortality (5, 47). Despite their results, most of our patients were included in the normal Na category, and no significant relationship between Na classification and Na level with the mortality status of patients and acid-base status was observed. Additionally, several paraclinical parameters, including Cl (48) and HCO3, were significantly related to Na classification and Na levels. Therefore, more studies are required to investigate the exact role of Na in COVID-19 progression.
The need for mechanical ventilation is another main outcome of COVID-19 patients. Its early management can directly affect COVID-19 patients’ health status. In this way, we considered mechanical ventilation another outcome of our study besides mortality status. Since significant changes in ventilation influence carbon dioxide elimination, it can lead to respiratory acid-base disorder (49, 50). Our results showed a statistically significant relationship between mechanical ventilation and pH. Similarly, Sjostrom et al. reported a significant association between pH and the need for mechanical ventilation and death (5). Sodium levels can be considered one of the important prognostic factors for mechanical ventilation. As a reason, pleural pressures and mechanical power increment cause an elevation in hemodynamic support, leading to proportional enhancement of Na, retention of fluid, and pulmonary edema (51). In the current study, a close but not significant relationship (P = 0.05) between Na levels and mechanical ventilation was revealed, which a significant link between the two variables is likely to appear just similar to a study by Sjostrom et al. and another study by Bihari et al. in case of larger sample sizes in future studies (5, 52). Furthermore, in a study by Cohen and Lambrinos, age was found to play a chief role in patients' outcomes and the need for mechanical ventilation. Our findings confirmed this claim but were accompanied by a positive relationship (53).
According to the need for mechanical ventilation, one of the predictive factors is an elevated level of LDH, like a study by Nicholson et al. (54). Another factor that may predict the need for mechanical ventilation is considered to be BUN. However, it is essential to note that BUN levels are not considered a primary deciding factor when considering mechanical ventilation. Other clinical and paraclinical parameters need to be gathered, including the patient's respiratory rate, oxygen saturation, ABG levels, mental status, and the underlying cause of respiratory failure (55, 56). Some other relationships between mechanical ventilation and paraclinical parameters, including P, Mg, lymphocyte count, PMN count, and O2 saturation, were investigated in our study.
Briefly, pH and Na levels are likely counted as the two main predisposing factors contributing to the need for mechanical ventilation in COVID-19 patients. Besides our significant results, most of the remaining findings were so close to significance, a lack of control group existed, and there was a small number of patients. Moreover, the confounding effect of medicine prescribed for patients concurrently suffering from diabetes, blood pressure, and kidney dysfunction must have been removed. Hereby, we suggest a cohort study without the abovementioned limitations, having the confounding effect of prescribed medicine for underlying diseases removed, to confirm the association between ICU-admitted patients’ mortality and the acid-base and electrolyte imbalances.
5.1. Conclusions
Acid-base imbalances, electrolyte abnormalities, and mechanical ventilation are some of the most important factors influencing the prognosis of COVID-19, and their identification and management can help prevent further disease progression. In conclusion, in accordance with underlying disease frequency, our study revealed that the majority of patients had diabetes. Also, mechanical ventilation showed a significant association with pH level and a close relationship with Na levels, demonstrating the importance of these factors and their predictive role in the need for mechanical ventilation. Furthermore, most of our patients suffered from respiratory alkalosis and mild hypokalemia. Despite no significance, most expired patients were from the respiratory alkalosis group. Therefore, we suggest considering respiratory alkalosis as a prognostic factor that can help to early identify high-risk patients and avoid severe health conditions.
We also revealed a significant connection between mortality and K levels, indicating K’s critical role in the prognosis of the disease. Intriguingly, K levels and pH levels displayed significant differences, which explains the possible effect of acid-base alteration on hypokalemia. Consequently, regarding the findings mentioned, it is proposed that patients’ follow-up regarding the acid-base and electrolyte balance status could help predict their status and assist in early diagnosis and accurate therapy to prevent the disease progression. Taking contradictory studies on COVID-19 into account, the conduction of complementary cohort studies is indeed proposed.