1. Background
Rheumatoid arthritis (RA) is an autoimmune disorder in which inflammatory mediators play a critical role in its pathogenesis, ultimately leading to joint and bone degeneration (1). The RA is a systemic disease characterized by inflammatory polyarthritis affecting both large and small joints, as well as systemic symptoms. Typical clinical signs of inflammatory arthritis, such as morning stiffness, gelling phenomena, and improvement with activity, are common in RA (2). In many patients, varying degrees of bone and joint degeneration or tendon sheath involvement result in deformities and functional loss. Small joints, such as those in the fingers, are more commonly affected, but larger joints, including the knees, hips, and shoulders, may also be involved. This chronic disease manifests as pain and inflammation in the joints of the fingers, wrists, and knees, with deformity and functional impairment often developing in more advanced stages (3, 4).
Approximately 0.4% to 1.26% of the global population suffers from RA, with prevalence increasing with age. The condition is three times more common in females than in males (1, 5). The progressive nature of RA significantly impacts patients' physical, social, and occupational activities, diminishes their quality of life, and often leads to anxiety, depression, and distress (6). Furthermore, the potential associations between RA and cardiovascular diseases or osteoporosis highlight its significance as a major health concern (6).
Management of RA typically involves the use of analgesics and non-steroidal anti-inflammatory drugs (NSAIDs). Disease-modifying anti-rheumatic drugs (DMARDs) aim to slow disease progression and prevent joint degeneration, while corticosteroids may be used for their anti-inflammatory properties. Non-steroidal anti-inflammatory drugs and steroids remain mainstays in RA treatment (7). However, the adverse effects of these drugs, such as gastrointestinal side effects associated with aspirin or water retention and abnormal fat distribution caused by steroids, pose serious challenges. Although aspirin can be substituted with paracetamol, its reduced efficacy and inability to halt tissue degeneration may lead to loss of joint mobility. Other drugs, including methotrexate, penicillin, gold compounds, and azathioprine, have also been utilized as DMARDs over the years. While these medications alleviate disease symptoms, they are often accompanied by significant side effects (7, 8).
There are concerns regarding the association of RA with other acute or chronic inflammatory disorders. Studies suggest that patients with RA have a twofold risk of myocardial infarction. Both typical and atypical risk factors may contribute to atherogenesis in RA, with insulin resistance being the most significant. Insulin resistance in RA may result from inflammatory processes and corticosteroid therapy. Similarly, the prevalence of diabetes mellitus is estimated to be 10 - 20% higher in patients with RA, making diabetes a predictive feature of cardiovascular disorders in RA (8, 9).
Insulin resistance is a critical component of metabolic syndrome, as defined by the World Health Organization. Evidence indicates that insulin resistance is more prevalent in patients with RA and lupus, predisposing them to a higher risk of cardiovascular diseases (10, 11). Modifiable risk factors, such as abdominal obesity, hypertension, and corticosteroid therapy, can be addressed to reduce the risk of insulin resistance in RA (11). Elevated levels of inflammatory factors in the body may be associated with insulin resistance, although their precise mechanisms remain unclear (10).
Anti-rheumatic drugs, NSAIDs, and corticosteroids are effective in alleviating pain, reducing inflammation, and slowing the progression of joint degeneration in RA. However, their use is accompanied by well-documented side effects (7, 9).
Insulin resistance encompasses a cluster of conditions, including hypertension, elevated blood insulin levels, abdominal fat accumulation, and increased serum lipid levels. Metabolic syndrome includes risk factors such as waist fat accumulation, abdominal obesity, hyperglycemia, low HDL levels, and high triglyceride and cholesterol levels. These factors collectively increase the risk of cardiovascular diseases, myocardial infarction, and diabetes mellitus (12, 13).
Given the significant impact of insulin resistance on patient outcomes and the prognosis of RA, early assessment and diagnosis could mitigate avoidable complications. This study aims to investigate the role of insulin resistance in patients with RA and clarify the status of this important comorbidity.
2. Objectives
To determine the insulin resistance in patients with RA based on the homeostasis model assessment of insulin resistance (HOMA-IR) levels.
3. Methods
3.1. Study Design
This descriptive, analytical, cross-sectional study was conducted to investigate insulin resistance in patients with RA who were referred to the rheumatology outpatient department (OPD) of the referral clinic in Qazvin province from January 27, 2020, to June 10, 2021. All patients included in the study had been under treatment with an unchanged regimen for at least two months. The participants were clinically examined, and indices of disease activity were assessed. Collected data included Body Mass Index (BMI), serum levels of triglycerides (TG), cholesterol, HDL, LDL, fasting blood sugar (FBS), and insulin. The HOMA test was conducted in the laboratory after 12 hours of fasting to assess insulin resistance. Anthropometric indices were also measured. The disease activity status was evaluated using the DAS-28 criteria.
3.2. Study Population and Sampling
This study employed convenience sampling to select participants. The study population comprised all patients diagnosed with RA who visited the OPD of the referral clinic in Qazvin, Iran, from January 27, 2020, to June 10, 2021. Patients were recruited based on inclusion and exclusion criteria using the convenience sampling method.
3.3. Diagnosis
Rheumatoid arthritis was diagnosed through physical examination, biochemical tests, and medical imaging, and confirmed based on the criteria established by the American College of Rheumatology (14).
3.4. Inclusion Criteria
A patient with RA, aged between 20 and 60 years (the age range of disease prevalence), willing to participate in the study, and providing a signed written consent form was included in the study.
3.5. Exclusion Criteria
Any patient with high blood pressure, smoking, pregnancy, or use of birth control pills (in women); suffering from chronic diseases such as kidney, respiratory, or heart diseases; taking estrogen or hormonal drugs; having a recent change in the dosage of RA treatment drugs; taking vitamin-mineral supplements in the past 2 months; undergoing surgery in the last 6 months; or having incomplete medical records was excluded from the study.
3.6. Measurable Outcome
The primary outcome of this study was the assessment of insulin resistance levels using the HOMA-IR.
3.7. Measurement Method
The HOMA-IR was used to evaluate the level of insulin resistance using the formula [fasting glucose (mmol/L) × fasting insulin (µU/mL)] (15). Anthropometric indices, including height (measured to a precision of 1 cm), weight (measured with a standard scale to a precision of 100 g), and abdominal and hip circumferences (measured with a plastic meter to an accuracy of 0.1 cm), were recorded. Disease activity was assessed using the disease activity score (DAS) based on 28 joints (DAS-28). This composite score is derived by pooling individual measures, including tender joint count (TJC), swollen joint count (SJC), and erythrocyte sedimentation rate (ESR). The DAS-28 score provides a comprehensive measure of disease activity for individual patients (16).
3.8. Statistical Analysis
The Shapiro-Wilk test was employed to evaluate the normality of the data. Descriptive statistics were used to present the findings. Chi-square and Fisher’s exact tests were applied to analyze categorical data. A paired t-test was used to compare means within groups, while an independent t-test was utilized to compare means between groups. The collected data were analyzed using SPSS statistical software version 18 (SPSS Inc., Chicago, IL, USA). A P-value of less than 0.05 was considered statistically significant.
3.9. Ethical Considerations
In this study, the principles of medical research ethics were followed in accordance with the Declaration of Helsinki and the guidelines of the National Committee on Ethics in Medical Research. The study proposal was approved by the medical ethics committee of Qazvin University of Medical Sciences (IR.QUMS.REC.1398.052). Only patients who were willing to participate and signed an informed consent form were included in the study.
4. Results
One hundred sixteen patients were enrolled in the study, including 103 (88.8%) females and 13 (11.2%) males. Their mean age was 54.8 ± 13.12 years (range: 26 - 84). The mean duration of the disease was 7.5 ± 7.01 years (range: 1 - 40). Other demographic and clinical characteristics of the patients are presented in Table 1. The mean blood glucose, cholesterol, and LDL levels were 11.4 mg/dL, 203.4 mg/dL, and 110.4 mg/dL, respectively.
| Variables | Min - Max | Mean ± SD |
|---|---|---|
| Height (cm) | 136 - 182 | 157.8 ± 8.6 |
| Weight (kg) | 49 - 103 | 70.9 ± 12.2 |
| BMI (kg/m2) | 18 - 48.9 | 28.6 ± 5.3 |
| FBS (mg/dL) | 70 - 319 | 100.4 ± 36.7 |
| Insulin (pmol/L) | 1 - 115 | 18.4 ± 14.6 |
| TG (mg/dL) | 30 - 373 | 122.3 ± 63.2 |
| Chol (mg/dL) | 129 - 350 | 203.4 ± 41.9 |
| LDL (mg/dL) | 43 - 218 | 110.4 ± 31.5 |
| HDL (mg/dL) | 30 - 123 | 57.9 ± 15.8 |
| AST (IU/L) | 8 - 51 | 20.1 ± 6.9 |
| ALT (IU/L) | 11 - 49 | 22.9 ± 9.5 |
Abbreviations: FBS, fasting blood sugar; TG, triglyceride; Chol, cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; AST, aspartate aminotransferase; ALT, alanine transaminase.
The drug regimen used by the patients is shown in Table 2. Most patients were taking prednisolone (98.1%) and methotrexate (92.6%), while slightly less than half were using SSZ.
| Drug | Frequency (%) |
|---|---|
| Steroids | 53 (98.1) |
| Methotrexate | 50 (92.6) |
| Sulfasalazine | 21 (40.4) |
| Hydroxychloroquine | 9 (17.3) |
| Azathioprine | 1 (2) |
| TNF-alpha inhibitors | - |
The mean insulin resistance was calculated at 4.4 ± 2.3, and the mean DAS was 3.7 ± 1.5. No statistically significant association was found between these two variables based on the Pearson correlation coefficient (r = 0.137, P = 0.188).
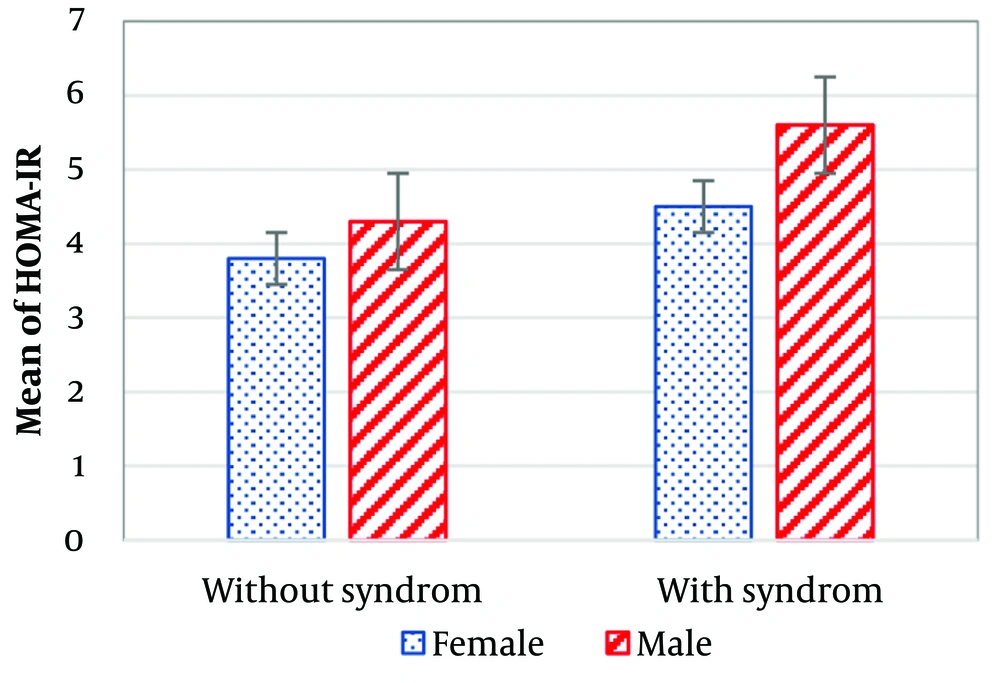
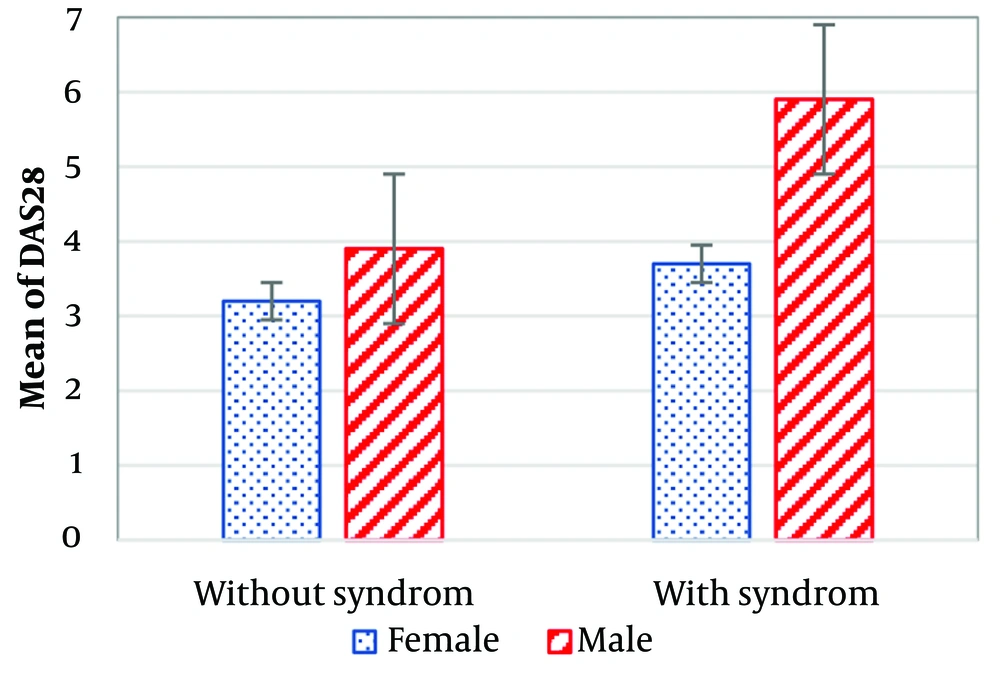
An independent sample t-test analyzing insulin resistance and disease activity by gender revealed a statistically significant difference (P < 0.0001). Male patients exhibited higher disease activity (5.3 ± 1.8) compared to females (3.5 ± 0.96). Similarly, the mean insulin resistance was higher in male patients (5.1 ± 2.4) compared to females (4.3 ± 2.2).
Of the studied patients, 37 (31.9%) did not have metabolic syndrome, while 79 (68.1%) did. The mean duration of RA was lower in patients with metabolic syndrome (7.1 ± 6.8 years) compared to those without metabolic syndrome (10.3 ± 7.4 years). Patients with metabolic syndrome had higher insulin resistance (4.6 ± 2.8) than those without metabolic syndrome (3.9 ± 2.3). However, the independent sample t-test did not show a statistically significant difference between the two groups (P = 0.188). Data on insulin resistance and disease activity in patients with and without metabolic syndrome are illustrated in Figures 1 and 2. Both variables were higher in male patients than in females.
Additionally, the independent sample t-test showed no statistically significant difference between insulin resistance and the drug regimen used by the studied patients.
5. Discussion
The current study aimed to evaluate the degree of insulin resistance in RA. One hundred sixteen patients with RA who attended a rheumatology clinic were selected after fulfilling the inclusion criteria. Their diagnoses were confirmed based on the criteria proposed by the US College of Rheumatology, and their drug regimens had remained unchanged during the previous two months. The collected data indicated some degree of insulin resistance in the studied patients, particularly in males and those with concomitant metabolic syndrome, although the findings were not statistically significant.
Rheumatoid arthritis can occur at any age, but its peak onset is typically between 30 and 50 years. The disease may progress to cause significant disability (2, 5). In a study conducted in the US, 35% of RA patients experienced functional disability within ten years of diagnosis. Socioeconomic status, cigarette smoking, and a positive family history are known risk factors for developing RA (17). Diagnosis requires the involvement of at least one painful and swollen joint, with involvement of one or more small joints further increasing the likelihood of RA. The presence of rheumatoid factor (RF), antibodies against citrullinated protein (ACPA) in the serum, and elevated CRP and ESR levels strongly support an RA diagnosis. Initial evaluations of patients should include CBC with WBC differential, as well as liver and renal function tests (7).
The findings of this study demonstrated that insulin resistance, as measured by the HOMA test, was elevated in patients with RA. Giles et al. similarly suggested that RA patients exhibit a substantial degree of insulin resistance (18). Additionally, Cai et al. reported that a high degree of insulin resistance in RA correlates with disease activity (19). These findings align with the results of the present study.
The mean insulin resistance and mean DASs in the current study were 4.4 ± 2.3 and 3.7 ± 1.5, respectively, suggesting a potential link between these variables. However, the association was not statistically significant. In contrast, other studies have reported a statistically significant association between these variables (13, 18, 19). The data on insulin resistance and disease activity in the present study varied by gender. The mean insulin resistance was 5.5 ± 3.3 in males and 4.3 ± 3.7 in females, while the mean DAS was 5.3 ± 1.8 in males and 3.5 ± 0.96 in females. These findings indicate that males had higher levels of both disease activity and insulin resistance.
More than two-thirds of the studied patients had metabolic syndrome in addition to RA. Interestingly, the mean duration of RA was shorter in patients with metabolic syndrome (7.1 ± 6.8 years) compared to those without metabolic syndrome (10.3 ± 7.4 years). Despite this, patients with metabolic syndrome exhibited higher levels of insulin resistance (4.6 ± 2.8) than those without metabolic syndrome (3.9 ± 2.3), although the difference was not statistically significant. Studies by Rostom et al. (13) and Cai et al. (19) have similarly reported a higher prevalence of metabolic syndrome in patients with RA compared to control groups. Their findings were statistically significant and varied depending on the criteria used for diagnosing metabolic syndrome. The present study aligns with these findings.
5.1. Conclusions
In patients with RA, the degree of insulin resistance increases in parallel with the level of disease activity and the severity of the condition, suggesting a relationship between various inflammatory mediators. Therefore, controlling disease activity and reducing inflammatory factors through appropriate therapy may also improve insulin resistance in RA.