1. Background
Fungal infections are a major concern in dental and medical settings, with Candida albicans being one of the most prevalent opportunistic pathogens. This yeast-like fungus is a normal commensal organism in the oral cavity, gastrointestinal tract, and genitourinary system, but it can become pathogenic under immunocompromised conditions or due to disruptions in the normal microbial flora (1). Candida albicans is responsible for various oral infections, including oropharyngeal candidiasis, denture stomatitis, and angular cheilitis, particularly in elderly patients, immunosuppressed individuals, and those undergoing prolonged antibiotic or corticosteroid therapy (2).
Dental clinics can serve as potential reservoirs for Candida species, with contamination occurring through direct contact with infected patients, aerosolized particles from dental procedures, or contaminated surfaces and instruments (3). The biofilm-forming capability of Candida species enhances their resistance to antifungal treatments and disinfection procedures, making infection control in dental environments particularly challenging (4). Studies have reported Candida species on frequently touched surfaces such as dental chairs, light handles, and impression trays, emphasizing the need for rigorous sterilization protocols (5).
Despite increasing awareness of fungal contamination in clinical settings, limited studies have specifically investigated the presence and persistence of multiple Candida species, including C. albicans, C. glabrata, and C. tropicalis on dental unit surfaces. Additionally, there is a lack of research exploring contamination patterns across different time points (before, during, and after clinical shifts) and the effectiveness of commonly used disinfectants (6). Understanding the relationship between surface contact, clinical activity levels, and the efficacy of disinfection can reveal critical risk factors in fungal transmission.
2. Objectives
This study aimed to (1) assess the prevalence of Candida species contamination on dental unit surfaces; (2) evaluate antifungal resistance patterns; and (3) analyze potential risk factors and propose effective infection control strategies. The findings will contribute to evidence-based improvements in infection control protocols in dental clinics.
3. Methods
3.1. Study Design and Setting
This cross-sectional study was conducted in the dental clinics of Zanjan University of Medical Sciences, Faculty of Dentistry, in 2023. The study aimed to assess Candida species contamination on frequently touched dental unit surfaces and evaluate the effectiveness of routine infection control measures.
3.2. Sample Collection
A total of 120 samples were collected from 12 active dental units. Sampling was conducted at three time points: Before the start of clinical sessions (pre-disinfection), during clinical activity, and after the end of sessions (post-disinfection). Samples were taken from four high-contact surfaces: Dental chair headrests, light handles, unit control panels, and saliva ejectors. Sterile cotton swabs moistened with 0.9% sterile saline were used to swab each surface for approximately 10 seconds in a standardized zigzag motion. Each swab was immediately transferred into sterile transport tubes containing Sabouraud dextrose broth (SDB) and sent to the microbiology laboratory for further analysis. Routine disinfection was performed using a 0.5% sodium hypochlorite solution. Surfaces were sprayed and left for 10 minutes before being wiped with sterile gauze, following manufacturer instructions. This procedure was repeated after each patient and at the end of each shift.
3.3. Fungal Culture and Identification
Samples were streaked onto Sabouraud dextrose agar (SDA) supplemented with chloramphenicol and incubated at 37°C for 48 hours. Colonies suspected to be Candida species were further identified based on: (1) Colony morphology (creamy white colonies with smooth surfaces); (2) gram staining; (3) germ tube test (positive for C. albicans); (4) Chromogenic agar differentiation (CHROMagar Candida).
3.4. Antifungal Susceptibility Testing
Antifungal susceptibility was tested using the disk diffusion method on Mueller-Hinton agar supplemented with 2% glucose and methylene blue. The antifungal agents tested included: (1) Fluconazole (25 µg); (2) Amphotericin B (10 µg); (3) Nystatin (100 units).
Zone diameters were measured and interpreted according to Clinical and Laboratory Standards Institute (CLSI) M44-A2 guidelines.
3.5. Statistical Analysis
Data were analyzed using SPSS Statistics release 27.0.1. The paired t-test and Wilcoxon signed-rank test were used to compare microbial loads before and after clinical sessions. A chi-square test was applied to analyze categorical data. Statistical significance was set at P < 0.05.
4. Results
4.1. Prevalence of Candida Species Across Different Departments
Out of 120 collected samples, Candida species were identified in 47 (39.2%) samples. The highest contamination was observed in the Prosthodontics Department (65.2%), followed by Endodontics (58.4%) and Periodontics (52.1%). The lowest prevalence was recorded in the Pediatric Dentistry Department (21.7%) (Table 1).
| Departments | Candida albicans (%) | Candida glabrata (%) | Candida tropicalis (%) | Total Contamination (%) |
|---|---|---|---|---|
| Prosthodontics | 38.6 (17/44) | 15.9 (7/44) | 10.7 (5/44) | 65.2 (29/44) |
| Endodontics | 32.4 (12/37) | 16.2 (6/37) | 10.8 (4/37) | 58.4 (22/37) |
| Periodontics | 29.2 (11/38) | 14.5 (5/38) | 8.4 (3/38) | 52.1 (19/38) |
| Pediatric dentistry | 12.3 (4/32) | 6.2 (2/32) | 3.2 (1/32) | 21.7 (7/32) |
Distribution of Candida Species Across Different Departments
4.2. Surface-wise Prevalence of Candida Species
Table 2 illustrates the distribution of Candida species across four high-contact surfaces of dental units before disinfection. The highest overall contamination rate was observed on chair headrests (72.5%), with C. albicans being the dominant species (38.3%), followed by C. glabrata (14.2%) and C. tropicalis (8.3%). Light handles also showed a relatively high contamination rate (65.8%), primarily due to the presence of C. albicans (32.5%), accompanied by C. glabrata (12.5%) and C. tropicalis (10.8%). Unit control panels exhibited a moderate level of contamination (44.2%), with C. albicans accounting for 28.3%. Finally, the lowest contamination was noted on saliva ejectors (32.5%), with C. albicans comprising 20% of the total contamination.
| Surface Type | Candida albicans (%) | Candida glabrata (%) | Candida tropicalis (%) | Total Contamination (%) |
|---|---|---|---|---|
| Chair headrests | 38.3 | 14.2 | 8.3 | 72.5 |
| Light handles | 32.5 | 12.5 | 10.8 | 65.8 |
| Unit control panels | 28.3 | 9.2 | 6.7 | 44.2 |
| Saliva ejectors | 20.0 | 7.5 | 5.0 | 32.5 |
Distribution of Candida Species Different Equipment
4.3. Comparison of Contamination Levels Before, During, and After Clinical Shifts
A significant increase in Candida contamination was observed during clinical activity, peaking after the shift (Table 3).
| Time Points | Contaminated Samples (%) | Mean ± SD (CFU/cm2) | P-Value |
|---|---|---|---|
| Before shift | 18.3 (22/120) | 6.8 ± 2.3 | - |
| During shift | 42.5 (51/120) | 17.6 ± 4.8 | 0.016 |
| After shift | 60.0 (72/120) | 28.4 ± 6.1 | 0.002 |
Contamination Levels Before, During, and After Clinical Shifts
To further clarify species dynamics during clinical activity, Table 4 summarizes the estimated prevalence of Candida species at each time point. C. albicans remained dominant in all stages, increasing steadily from 12.5% before shifts to 41.6% after shifts.
| Time Points | Total Positive Samples | Candida albicans (%) | C. glabrata (%) |
|---|---|---|---|
| Before shift | 22 | 12.5% | 3.3% |
| During shift | 51 | 29.2% | 7.5% |
| After shift | 72 | 41.6% | 10.0% |
Estimated Prevalence of Candida Species Across Time Points
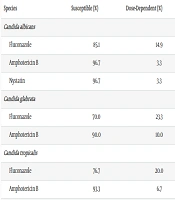
4.4. Antifungal Susceptibility Testing
Antifungal susceptibility testing revealed that 85.1% of C. albicans isolates were susceptible to fluconazole, while 14.9% showed dose-dependent susceptibility. Amphotericin B and nystatin demonstrated high efficacy, with 96.7% susceptibility (Table 5).
| Species | Susceptible (%) | Dose-Dependent (%) | Resistant (%) |
|---|---|---|---|
| Candida albicans | |||
| Fluconazole | 85.1 | 14.9 | 0.0 |
| Amphotericin B | 96.7 | 3.3 | 0.0 |
| Nystatin | 96.7 | 3.3 | 0.0 |
| Candida glabrata | |||
| Fluconazole | 70.0 | 23.3 | 6.7 |
| Amphotericin B | 90.0 | 10.0 | 0.0 |
| Candida tropicalis | |||
| Fluconazole | 76.7 | 20.0 | 3.3 |
| Amphotericin B | 93.3 | 6.7 | 0.0 |
Antifungal Resistance Patterns of Candida Species Isolated from Dental Unit Surfaces
4.5. Correlation Between Contamination and Clinical Activity
A strong positive correlation (R = 0.74, P < 0.001) was observed between the number of patient visits per unit and the level of Candida contamination. Units with a higher patient flow exhibited significantly higher contamination levels.
4.6. Effectiveness of Routine Disinfection Protocols
Despite routine disinfection, 39.2% of surfaces remained contaminated post-disinfection, suggesting that current sterilization protocols may not be fully effective against Candida species. The highest residual contamination was found on dental chair headrests (49.6%) and light handles (42.3%) (Table 6).
| Surface Type | Candida albicans (%) | Candida glabrata (%) | Candida tropicalis (%) | Total Post-Disinfection Contamination (%) |
|---|---|---|---|---|
| Chair headrests | 27.5 | 12.1 | 10.0 | 49.6 |
| Light handles | 24.8 | 10.5 | 7.0 | 42.3 |
| Unit control panels | 15.0 | 6.2 | 4.0 | 25.2 |
| Saliva ejectors | 8.0 | 4.0 | 2.0 | 14.0 |
| Overall average | - | - | - | 39.2 |
Estimated Prevalence of Candida Species Across Time Points
5. Discussion
The findings of this study reveal a significant increase in Candida contamination on dental unit surfaces during and after clinical shifts, emphasizing the potential risk of fungal transmission in dental settings. The contamination rate increased from 18.3% before shifts to 60.0% after shifts (P < 0.001), with the highest fungal loads observed on dental chair headrests (72.5%) and light handles (65.8%). These results align with previous research indicating that high-contact surfaces in dental clinics serve as reservoirs for microbial persistence (7).
This trend highlights several risk factors contributing to contamination, including: (1) High patient turnover during peak clinical hours; (2) aerosol generation during dental procedures such as ultrasonic scaling and high-speed drilling; (3) inadequate surface coverage during routine disinfection; and (4) failure to change gloves or disinfect hands between patient interactions. These factors may collectively enhance the survival and spread of fungal pathogens on surfaces (8).
The bioaerosol mechanism has been documented by Gallagher et al. and others, where fungal spores can remain airborne for extended periods and deposit onto equipment surfaces (8). Our results support this, particularly due to elevated contamination levels during and after active clinical sessions (9).
Several studies have reported similar patterns of Candida contamination in dental environments. De Almondes et al. found that C. albicans was present on 58% of dental chairs, particularly in departments with high patient turnover (10). Similarly, Pandey et al. demonstrated that unit control panels and light handles harbored the highest fungal loads, reinforcing the need for enhanced sterilization measures (11). Our findings are also consistent with those of Mobin et al., who observed persistent fungal contamination in 42% of dental units despite routine cleaning protocols (12). In contrast to Turner and Butler, who reported C. tropicalis as dominant in some dental environments, our study found C. albicans as the predominant species, followed by C. glabrata and C. tropicalis, indicating a possible regional variation or demographic influence on species distribution (13). This variation may stem from regional differences, patient demographics, or evolving antifungal resistance profiles, which warrant further investigation.
The persistence of Candida contamination post-disinfection (39.2%) is likely attributed to the biofilm-forming ability of the isolates, rendering routine hypochlorite disinfection insufficient in eliminating all fungal cells. Biofilms enhance resistance by forming protective extracellular matrices and promoting cell aggregation, making conventional cleaning protocols less effective (14).
Therefore, improved infection control strategies are urgently needed. Based on our findings and previous literature, we recommend the following (15-17): (1) Increasing the frequency of surface disinfection, especially between patients; (2) utilizing antifungal-effective disinfectants (e.g., hydrogen peroxide-based or chlorhexidine-gluconate solutions); (3) introducing UV-C light sterilization for high-touch surfaces; (4) applying disposable plastic barriers to chair headrests, light handles, and control panels; (5) reinforcing hand hygiene protocols and mandatory glove changes between patients.
These combined interventions can significantly reduce fungal load and limit the risk of cross-infection, particularly for immunocompromised patients.
Despite its strengths, this study has several limitations. The single-center study design limits the generalizability of the findings, and environmental variables such as room ventilation, humidity, and temperature were not recorded, which may influence fungal persistence (18). Furthermore, only conventional culture-based methods were used for species identification; molecular confirmation was not performed, which could underestimate species diversity.
Additionally, the identification of Candida species relied on culture-based methods rather than molecular techniques, which could lead to underestimation of species diversity (19). Future research should focus on multi-center studies, molecular identification methods, and the evaluation of novel antifungal disinfectants (20).
In conclusion, the results of this study highlight the need for stricter infection control policies to reduce Candida contamination on dental unit surfaces. Implementing enhanced disinfection protocols, improving personal protective measures, and conducting routine microbial surveillance are critical steps toward minimizing the risk of fungal transmission in dental settings (21).
5.1. Conclusions
This study highlights the significant presence of Candida species on dental unit surfaces, with a clear increase in contamination from 18.3% before shifts to 60.0% after shifts (P < 0.001). The most contaminated surfaces were chair headrests (72.5%) and light handles (65.8%), representing high-risk contact points in clinical settings.
Despite routine disinfection using sodium hypochlorite, 39.2% of surfaces remained contaminated post-cleaning, indicating that current sterilization protocols may be insufficient, particularly against biofilm-forming species such as C. albicans. Based on the study’s findings, we strongly recommend the following to minimize fungal transmission in dental clinics: (1) More frequent and rigorous surface disinfection, especially of high-contact areas; (2) use of UV-C light or enhanced antifungal disinfectants; (3) implementation of plastic barrier coverings; (4) reinforced staff training in infection control, including hand hygiene and glove protocols.
By adopting these measures, dental care environments can significantly reduce the risk of cross-infection, thus enhancing the safety of both patients and healthcare workers.
Antifungal susceptibility testing revealed that 85.1% of C. albicans isolates were susceptible to fluconazole, while 96.7% showed sensitivity to amphotericin B and nystatin. These findings suggest that while antifungal agents remain largely effective, preventive strategies must focus on limiting surface contamination rather than relying solely on drug susceptibility.
5.2. Recommendations
To minimize fungal transmission in dental settings, the following strategies are recommended: (1) More frequent and rigorous surface disinfection, particularly on high-contact areas; (2) use of advanced sterilization techniques, such as UV-C light disinfection; (3) implementation of microbial surveillance programs to monitor contamination trends; (4) enhanced training for dental personnel on proper hand hygiene and equipment sterilization.
By incorporating these measures, dental clinics can significantly reduce the risk of fungal contamination, improving both patient and practitioner safety.