1. Introduction
Rhabdomyolysis (RM) is a condition characterized by the disintegration and necrosis of muscle tissue, along with the release of intracellular material into the bloodstream (1). Approximately 13 - 46% of patients with RM have been reported to develop acute kidney injury (AKI) (1). Therapeutic plasma exchange (TPE), an intensive treatment for refractory myasthenia, faces certain limitations in several developing nations (2, 3). Numerous studies have established the anti-inflammatory effects of superoxide dismutase (SOD). Furthermore, a considerable proportion of healthcare practitioners in our nation advocate for the use of SOD in managing AKI associated with RM, despite a lack of evidence (4). While evidence suggests a possible link between reactive oxygen species (ROS) and the development of AKI in RM cases, the precise association between SOD and AKI in the context of RM has not been thoroughly explored or documented in published scientific literature (1, 4).
Rhabdomyolysis is a clinical condition characterized by the breakdown of skeletal muscle tissue, resulting in the release of muscle cell constituents into the bloodstream. These constituents include electrolytes, myoglobin, and several sarcoplasmic proteins (5-8). It can manifest due to various pathological factors, including trauma, physical exertion, medicinal therapies, and infectious pathogens. However, it is crucial to recognize that in the pediatric population, RM is primarily linked to infection and genetic disorders. Approximately 50% of individuals diagnosed with RM exhibit symptoms such as myalgias, weakness, and dark-colored urine. The AKI is a frequently recognized and significant consequence of kidney impairment.
Effective resource management may necessitate the application of TPE due to the limitations of continuous kidney replacement therapy (CKRT) in rapidly eliminating molecules associated with RM, given their molecular size (3, 9). Some developing countries face limitations in the use of TPE, as the necessary tools are available only in major cities, and national insurance is not yet established. This study presents a case of a juvenile patient who experienced AKI as a consequence of RM.
2. Case Presentation
A 16-year-old male adolescent presented with a complaint of reduced urine output five days prior to hospital admission. The severity of this condition escalated until the day before hospitalization, at which point the patient experienced complete urinary retention. The urine was deep brown, resembling tea. The patient also presented with bilateral leg edema, lower back discomfort radiating to the thighs and calves for five days prior to hospitalization, which subsequently subsided, and an overall sense of weakness. There were no symptoms such as fever, shortness of breath, cough, runny nose, convulsions, or altered consciousness.
Due to these complaints, the patient was admitted to the pediatric ward at Hasan Sadikin General Hospital. The patient had a history of engaging in vigorous physical exercise three days per week prior to admission. During this period, he consumed only 100 mL of water per day. Despite experiencing fatigue and dehydration, he completed all tasks during this time. There was no prior history of similar complaints, kidney disease, or cancer, nor did any report occurrences of respiratory symptoms or bodily discomfort in the preceding week.
Physical examination revealed elevated blood pressure (148/85 mmHg), abdominal distention with normal bowel sounds, ascites, and non-pitting edema in the bilateral lower extremities. Urine output was 200 mL over 24 hours, equivalent to approximately 0.1 mL/kg body weight/hour.
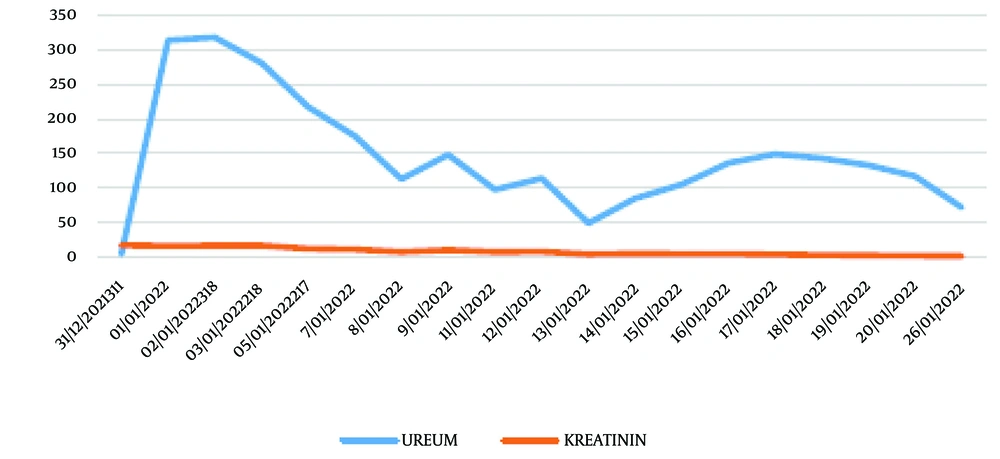
Laboratory findings (Table 1) showed a hemoglobin level of 11.3 g/dL, hematocrit of 35%, leukocyte count of 12,790/mm3, platelet count of 225,000/mm3, and a leukocyte differential count of 0/1/1/80/6/12. The mean corpuscular volume (MCV) was 53.8 fL, mean corpuscular hemoglobin (MCH) was 17.4 pg/cell, and mean corpuscular hemoglobin concentration (MCHC) was 32.3 g/dL. Serum urea was 314 mg/dL, and serum creatinine was 16.55 mg/dL. The estimated glomerular filtration rate (eGFR) was 7.57 mL/min/1.73 m2. Sodium was 120 mEq/L, potassium was 3.8 mmol/L, calcium was 4.00 mg/dL, and albumin was 2.22 g/dL. Liver function tests revealed a serum glutamic oxaloacetic transaminase (SGOT) level of 319 U/L and a serum glutamic pyruvic transaminase (SGPT) level of 379 U/L. The lipid profile showed a total cholesterol level of 117 mg/dL, high-density lipoprotein (HDL) level of 23 mg/dL, low-density lipoprotein (LDL) level of 66 mg/dL, and triglyceride level of 173 mg/dL. Creatinine kinase (CK) was measured at 6,600 mcg/L.
| Laboratory Findings | Dates | |||||
|---|---|---|---|---|---|---|
| 1/1/22 | 2/1/22 | 5/1/22 | 7/1/22 | 8/1/22 | 9/1/22 | |
| Hemoglobin | 11.3 | 11.5 | - | - | 8.4 | - |
| Hematocrit | 35.0 | 34.7 | - | - | 25.7 | - |
| Erythrocyte | 12.0 | - | - | - | - | - |
| Leucocyte | 12.790 | 13330 | - | - | 18.960 | - |
| Thrombocyte | 225.000 | 338000 | - | - | 439.000 | - |
| DC | 0/1/1/80/6/12 | 0/0/0/86/5/9 | - | - | 0/1/2/82/6/9 | - |
| Urea | 314.0 | 318 | 217 | 175 | 113 | 148 |
| Creatinine | 16.55 | 16.92 | 11.9 | 11.07 | 7.32 | 10.25 |
| eGFR | 7.57 | 8 | - | - | - | 8.7 |
| Na | 120 | 120 | 123 | 123 | 127 | - |
| K | 3.8 | 5.2 | 4.5 | 5.0 | 4.6 | - |
| Ca | 4 | 5.05 | 4.66 | 4.70 | 5.27 | - |
| CKMB | - | 423 | - | - | 95 | - |
| CK | - | - | 3953 | - | 2501 | - |
Abbreviations: DC, differential count; eGFR, estimated glomerular filtration rate; CK, creatinine kinase; CKMB, creatinine kinase myocardial band.
Initial urine findings (Table 2) in the emergency department revealed a pH of 6.5, protein level of +3, presence of erythrocytes at +3, and detection of bacteria upon microscopic analysis. A subsequent urine analysis showed a specific gravity of 1.020, absence of protein, and presence of erythrocytes at +3. The chest X-ray indicated evidence suggestive of cardiomegaly. Ultrasound examination revealed no discernible abnormalities in the kidneys and bladder.
| Urinalysis | Date (1/1/22) |
|---|---|
| Color | Brown |
| Protein | +3 |
| Retrofit | 3+ |
| Eritrosit | > 50 |
| Leukosit | 5 - 9 |
| Epitel | 1 - 3 |
| Bakteri | Positive |
| Silinder | Negative |
| Crystal | Negative |
Based on the available data, the patient’s diagnosis was AKI attributed to rhabdomyolysis, accompanied by stage II hypertension, increased liver enzymes, and an electrolyte imbalance characterized by hyponatremia. The patient received hydration therapy at a rate of 2 liters per m2 per day, in addition to meeting a daily caloric need of 3100 kilocalories. The patient was administered intravenous furosemide, a diuretic, at a dosage of 2 × 40 mg. Additionally, captopril, an angiotensin receptor blocker, was administered orally at a dosage of 2 × 12.5 mg. Hydration management was performed carefully, involving periodic intravascular observation using point-of-care ultrasound (POCUS) with a two-dimensional ultrasound machine, by establishing the inferior vena cava to aortic ratio (IVC/Ao) to determine hypervolemia or euvolemia (10, 11).
On the third day of hospitalization, the patient’s serum urea level was measured at 318 mg/dL, creatinine level at 16.92 mg/dL, sodium level at 114 mEq/L, potassium level at 6.2 mmol/L, and creatine kinase (CK) level at 423 mcg/L. The electrocardiographic examination revealed elevated T waves. The patient underwent treatment for hyperkalemia, specifically with the administration of oral resin at a dosage of 3 × 5 grams, as well as calcium gluconate at a concentration of 10%. Additionally, the hyponatremia was successfully managed with the administration of a 3% intravenous NaCl solution over 24 hours.
The patient underwent acute hemodialysis treatment with the following prescription: A duration of three hours, a blood flow rate of 150 mL/minute, a dialysate flow rate of 400 mL/minute, and a target ultrafiltration volume of 2000 mL. The patient underwent hemodialysis on six occasions. Additionally, the patient received therapy with the administration of superoxide dismutase (GlySODin®) tablets twice daily (1 tablet equivalent to 250 IU of superoxide dismutase), Curcuma syrup (Curbexon®) at a dosage of 15 mL (each 5 mL contains curcuma xanthorrhiza 10 mg) once daily, amlodipine once daily at a dosage of 10 mg, and vitamin C at a dosage of 500 mg twice daily. The symptoms and indications exhibited improvement, with the absence of both edema and dyspnea.
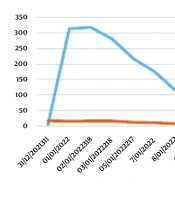
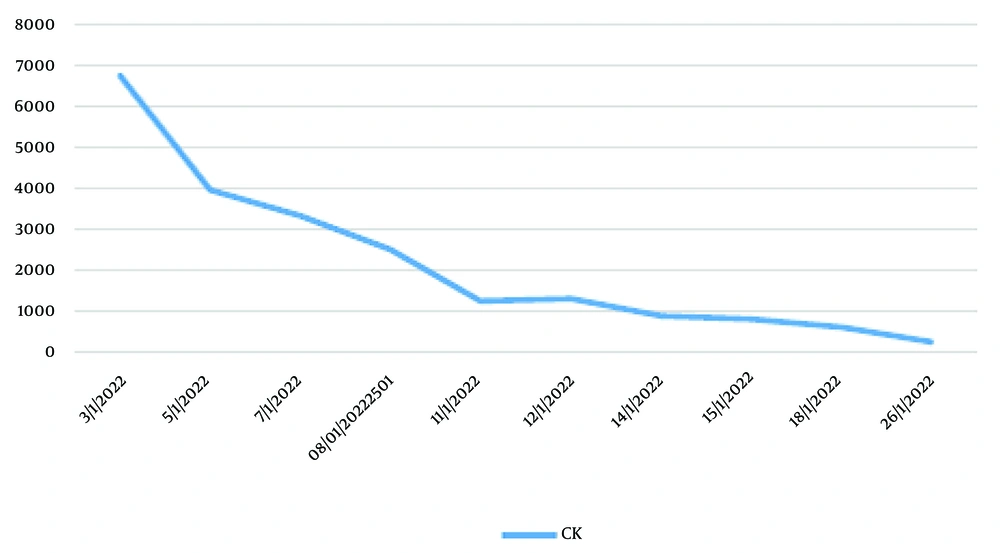
Following the hemodialysis procedure, there was observed improvement in the levels of serum urea, creatinine, and electrolytes. The patient had a two-week treatment period and was subsequently discharged, exhibiting notable improvement in serum urea, creatinine, and CK levels, which measured at 117 mg/dL, 2 mg/dL, and 610 U/L, respectively. The individual was assessed at the nephrology outpatient facility, which yielded positive findings on the physical examination. Additionally, there was improvement observed in the levels of serum urea, creatinine, and CK, measuring 72 mg/dL, 1.36 mg/dL, and 251 mcg/L, respectively. The reduction of serum CK was challenging, and certain technical difficulties with the machine prevented the execution of certain TPE procedures. Consequently, the patient was administered SOD. Figure 1 illustrates the observed decline in serum CK levels subsequent to the treatment with SOD.
During the process of hydration therapy, the presence of AKI resulted in the development of congestive heart failure and pulmonary edema. Therefore, hemodialysis (HD) was conducted as a form of kidney replacement therapy (KRT) to regulate the intravascular volume. The patient’s care involved intravascular volume management through hydration with HD as a supportive measure, along with conservative management of AKI, including antihypertensive medications, blood transfusion, dietary adjustments, and supplementation with SOD and curcuma. The clearance of metabolites gradually improved with conservative AKI treatment and SOD supplementation (Figure 2).
3. Discussion
Kidney involvement as a consequence of RM is a significant pathological disorder that necessitates intensive therapeutic interventions. Existing scholarly research indicates that the causes and clinical presentations of pediatric patients with RM exhibit notable distinctions compared to their adult counterparts. A prior investigation in adults revealed that the occurrence of respiratory syncytial virus in adult patients may be attributed to factors including alcohol use, muscle compression, severe muscle injury, metabolic disorders, and hypothermia (12). The current instance demonstrated that the occurrence of RM may be attributed to muscle fatigue and chronic dehydration. Nevertheless, it is imperative to consider the possibility of silent viral infection in these patients. According to Wu et al., viral myositis was identified as the leading cause of pediatric RM, accounting for almost 50% of cases. The second and third most significant characteristics associated with risk management were identified as physical exertion and seizure disorder, respectively (13). In the study by Kim et al. (as cited by Lim et al.), respiratory tract infection (n = 9, 15%) and seizure (n = 7, 11.6%) were identified as primary variables associated with RM in the pediatric population (14). Numerous documented examples of exercise-induced RM occur independently of genetic abnormalities. Most cases have been observed within groups engaged in athletic activities, military recruitment, and running (15). Exercise-induced RM may be impacted by factors such as increased humidity levels, anticholinergic drugs, hypokalemia, and performance-enhancing chemicals (16). Additionally, eccentric contractions, which occur when muscles generate tension, particularly during activities like downhill running, have a greater likelihood of causing muscular damage compared to concentric contractions, which primarily entail muscle flexion (16). Hematuria among individuals who participate in raving events has been recorded in instances where prolonged dancing is involved, leading to subsequent development of RM and hematuria. The prevalence of this phenomenon seems higher when amphetamines are used concurrently (16).
The individual refrained from consuming anticholinergic medication and instead participated in intensive physical training, i.e., military activities designed to recruit mountaineering personnel. In physiological settings, the contraction of skeletal muscle cells necessitates a neural impulse originating from a voluntary mechanism. Following this, the propagation of the neural signal takes place within a narrow cellular boundary referred to as the sarcolemma (17). In healthy myocytes, the sarcolemma is endowed with several pumps that play a crucial role in regulating the complex mechanism of cellular electrochemical gradients (17). The Na-K-ATPase enzyme serves a pivotal function in enabling the translocation of sodium and potassium ions. The primary function of the sarcoplasmic reticulum is to act as a storage site for calcium ions, which are subsequently released during muscle contraction (7).
The depletion of adenosine triphosphate (ATP) resulting from RM has been identified as a consequential effect, which subsequently leads to the malfunctioning of cell membrane pumps. The sodium extrusion mechanism is impeded, whereas the calcium efflux from the cell is impeded (18). The activation of cytolytic enzymes, such as hydroxylases, proteases, nucleases, and others, occurs in the sarcoplasm when there is a prolonged elevation in calcium levels (19). Impaired cell organelles, including mitochondria, lead to a series of effects including a gradual decrease in ATP levels, the production of ROS, and subsequent cellular destruction (19). The malfunction of cells results in the liberation of several chemicals, such as potassium, phosphates, myoglobin, creatine kinase (CK), lactate dehydrogenase, and aldolase, into the circulatory system. This pathological state is frequently denoted as RM and is distinguished by certain clinical presentations (7).
The etiology of AKI resulting from RM is believed to be triggered by myoglobin, which serves as the nephrotoxic agent causing kidney dysfunction. Myoglobin can be identified in urine at low quantities of less than 5 μg/L, whereas amounts exceeding 20 μg/L are considered indicative of myoglobinuria according to diagnostic criteria. Myoglobin, a protein that can be reabsorbed from the glomerular filtrate and broken down within proximal tubule cells, has been identified as a contributing factor in the occurrence of AKI in cases with myoglobinuria. The kidney toxicity induced by myoglobin is commonly described in terms of three distinct processes: Kidney vasoconstriction, intratubular cast formation, and direct myoglobin-induced toxicity to kidney tubular cells (7).
The accurate identification of RM typically necessitates a comprehensive collection of patient history and a heightened clinical suspicion. The diagnosis is strongly indicated when there is a significant increase in CK levels, above four to five times the upper limit of normal, and when the urine dipstick test detects blood in the absence of red blood cells. The differentiation between myoglobinuria, characterized by normal coloration of serum, and hemoglobinuria, characterized by brown or red serum, can be facilitated through the examination of serum color (16). The confirmation of the diagnosis can be achieved through the quantification of myoglobin levels in either serum or urine samples. However, it is important to note that obtaining the results of these tests may require a waiting period of several days. The elevation in serum myoglobin occurs prior to the elevation of CK. The latter often exhibits an increase approximately 12 hours subsequent to the provocation and reaches its highest point during a span of 2 to 3 days. The limited duration of myoglobin’s presence in serum, typically lasting between 1 to 3 hours, renders plasma myoglobin levels an unreliable indicator. Consequently, the timing of assays may lead to false-negative outcomes (14).
Inflammatory myopathies, namely polymyositis, dermatomyositis, and inclusion body myositis, may exhibit elevated levels of CK. However, these levels are typically not as pronounced as those shown in muscular dystrophies, such as Duchenne muscular dystrophy. The manifestation of myoglobinuria becomes apparent when the concentration of myoglobin in the urine surpasses 250 μg/mL, indicating the degradation of over 100 g of muscular tissue (20). If there is a suspicion of exercise-induced RM, additional investigations may be necessary, such as a non-ischemic forearm exercise test, muscle biopsy, and genetic testing (16).
The management of RM often encompasses three key elements: Cessation of any ongoing skeletal muscle injury, mitigation of AKI, and prompt detection of potentially critical consequences. The implementation of targeted interventions to mitigate the occurrence of persistent muscular injury will be contingent upon the underlying cause of RM (5).
The central emphasis in the management of kidney injury is primarily directed towards the reduction of kidney damage (5). The early and proactive provision of fluids to restore adequate kidney perfusion and promote urine flow is widely recognized as the key strategy for preventing and managing AKI resulting from RM (5). The administration of intravenous fluid (IVF) has the capacity to attenuate the concentration of nephrotoxic agents and enhance movement inside kidney tubules. This procedure can impede the accumulation of myoglobin and other deleterious substances within the renal parenchyma (5). There is considerable debate about the specific nature and quantity of fluid, as well as the utilization of diuretic agents. In cases where a patient is suspected or diagnosed with RM, it is imperative to promptly deliver IVF as an initial bolus (7). Isotonic saline solution is commonly favored due to its widespread availability and absence of potassium content. Administering IVF promptly before or during the extrication of crush victims in catastrophic circumstances may be beneficial in mitigating kidney problems. The IVF administration is maintained until the resolution of RM or until the occurrence of oliguric AKI restricts further fluid infusion (7). During the initial 24-hour period following presentation, a range of 3 L to 24 L has been shown to be effective. A target range of 6 to 12 liters within a 24-hour period is considered reasonable, provided that problems resulting from excessive fluid volume are well prevented. Historically, it has been advised to set urine output targets at 2 mL per kilogram of body weight each hour (7).
The efficacy of CKRT for the treatment of RM was evaluated in a Cochrane review conducted in 2014. This analysis included three randomized controlled trials (RCTs) with a total of 101 patients. The primary outcomes analyzed were mortality, kidney outcome, and hospital length of stay. No statistically significant difference in mortality or adverse events was observed, although there was a suggestion of a shorter hospital length of stay in patients who underwent preventive CKRT. Hemodialysis is indicated in cases of hyperkalemia, metabolic acidosis, volume overload, and azotemia. The findings of a comprehensive study and synthesis of existing research on KRT modalities for AKI in intensive care unit (ICU) patients indicate that no one KRT modality demonstrates a clear advantage in terms of short-term patient survival or dependence on dialysis (21).
The recommendation is against the use of alkalinization for the prevention of AKI generated by radiocontrast media (21). A randomized controlled trial conducted across many centers in France examined the efficacy of sodium bicarbonate compared to saline in preventing contrast-induced AKI in critically ill patients. The study identified no significant disparity in outcomes relevant to patient well-being (22). The PRESERVE experiment involved the randomization of 5177 high-risk patients scheduled for angiography. These patients were divided into two groups using a 2-by-2 factorial design. One group received oral acetylcysteine and intravenous saline for 5 days, while the other group received intravenous bicarbonate. There was no observed disparity in mortality rates, requirement for KRT, or sustained deterioration in kidney function (23). The use of antioxidant therapy among the general population in the ICU is controversial, as evidenced by inconsistent findings in systematic reviews (21). Previous research supports the use of SOD in AKI related to RM, with the mechanism of controlling ROS signaling; therefore, further cell and tissue damage can be controlled. The less tissue damage there is, the easier it is to manage myoglobin, thus facilitating HD therapy to remove myoglobin (1, 4).
Nevertheless, the current patient exhibited a notable enhancement in clinical condition as seen by the declining serum CK level and raised liver enzyme following the administration of SOD and curcuma. Superoxide dismutase seems important as an adjuvant in the management of AKI so that it does not develop into acute kidney disease (AKD), which can then lead to chronic kidney disease (CKD). If CKD progresses, then in developing countries where chronic ambulatory peritoneal dialysis (CAPD) is not yet established, there will be a decrease in the quality of life of children and even mortality (24).
3.1. Conclusions
In this study, we present a case of AKI caused by RM. Heavy physical exertion and reduced hydration intake are considered significant contributing causes. The resolution of the illness was achieved with the implementation of either conservative management or KRT, IVF administration for hydration, and the administration of SOD with curcuma. The main therapeutic approach for management is the administration of fluids to maintain hydration levels. Additionally, in cases where there is excessive fluid accumulation leading to pulmonary edema or congestive heart disease, KRT may be necessary. The application of SOD and curcuma as an adjuvant to HD and hydration therapy resulted in a rapid improvement in RM. Baseline CK was approximately 6,600 mcg/L and decreased to 1,000 U/L after 8 days of HD, hydration, and a combination of curcuma and SOD administration. However, this case report has significant limitations, which cannot dismiss the bias of whether the decreasing CK and urea levels were primarily due to HD, hydration, curcuma, or SOD. Nevertheless, the good results from the combination of these modalities provide positive outcomes for the patient, preventing the occurrence of AKI and even CKD, as the patient recovered completely.
In addition, this case report also has limitations, as urine myoglobin tests are not available in the hospital, and genetic/metabolic workups, such as McArdle disease and mitochondrial disorders, could not be performed. A future research endeavor of value would be a prospective cohort study examining the potential benefits of SOD and curcuma in the management of RM-related AKI.