1. Context
The gut microbiome, composed of trillions of microorganisms, is crucial for health, aiding in digestion, immune function, and metabolism. However, imbalances can lead to inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and metabolic disorders. Gut bacteria are also associated with diseases such as colorectal cancer (CRC), cardiovascular issues, and neurological disorders. Treatments such as fecal microbiota transplantation (FMT) and probiotics are being studied, highlighting the vital role of the microbiome in health and its ability to restore balance (1). This review synthesizes current evidence on the role of gut microbiota in inflammation, focusing on mechanistic insights and therapeutic potential, and addressing specific questions:
1. How does gut dysbiosis contribute to the pathogenesis of inflammatory (e.g., IBD), autoimmune (e.g., MS, T1D), and metabolic diseases (e.g., obesity, CVD)?
2. What are the key microbial metabolites (e.g., SCFAs, TMAO) and pathways linking gut microbiota to extra-intestinal diseases (e.g., neurodegenerative disorders)?
3. How can microbiome-targeted therapies (e.g., FMT, probiotics, dietary interventions) modulate disease outcomes?
Recent studies reveal gaps in causality evidence, and we suggest future research directions for clinical translation.
2. Methods
2.1. Search Strategy and Selection Criteria
The systematic literature search was conducted to identify studies on the gut microbiota's role in inflammation, autoimmunity, and multi-organ diseases. Our systematic approach included:
1. Engines & databases: PubMed/MEDLINE, Google Scholar, Web of Science with Keywords like Gut Microbiome, IBD, IBS, Dysbiosis, GERD, CRC.
2. Search strategy: Searches review articles from 2010 to 2025.
3. Screening process: Initially, about 90 articles were reviewed. The information from 54 articles was utilized.
4. Selection criteria:
• Inclusion: Original research articles, Systematic reviews and meta-analyses, Clinical trials, Studies with clear methodology and outcomes, Publications in peer-reviewed journals.
• Exclusion: Review articles with <10 subjects
The search strategy was designed to be comprehensive yet focused on our research questions about microbiota-disease interactions and therapeutic potential.
2.2. Gut Microbiota and Dysbiosis
The gut microbiome, a community of microorganisms in the digestive system, is crucial for digestive health and disease progression. It evolves throughout life, with imbalances (dysbiosis) linked to disorders like IBD, IBS, and cancers, influenced by genetics and environmental factors (2).
2.3. Factors Influencing the Balance of the Normal Microbiota Populations
2.3.1. Environmental Factors
Environmental factors like lifestyle choices, living conditions, antibiotic use, and exposure to harmful substances impact health. Overuse of antibiotics harms gut bacteria, urban living decreases microbial diversity, and pollutants impact the microbiome (3).
2.3.2. Genetic Factors
Genes significantly influence gut bacteria through their effects on the immune system and gut health. Variants in genes like NOD2 and IL23R can increase the risk of Crohn's disease and IBD. MUC2 mutations raise colitis risk, while metabolism-related genes such as FTO and ABCB11 impact bacterial growth. Variations in LCT are associated with beneficial Bifidobacterium for lactose digestion. Understanding these factors is crucial for evaluating disease risk and developing targeted IBD therapies (4).
2.4. The Gut Microbiota and Its Role in Inflammation and Immune Regulation, and Surrogate Factors to Detect Theses Condition
C-reactive protein (CRP) is an inflammatory marker produced by the liver in response to cytokines like TNF-α and IL-6. Elevated CRP levels indicate inflammation associated with conditions like IBS, (IBD, and CRC. While some IBS patients may show increased CRP, it's more typically linked to IBD and chronic inflammation, which elevate cancer risk (5). The gut microbiome significantly influences CRP levels, with pathogenic bacteria increasing CRP, while beneficial metabolites, particularly short-chain fatty acids (SCFAs), may lower it. A healthy gut microbiome is vital for regulating immune responses and preventing chronic inflammation, as dysbiosis can lead to broader health issues (6).
2.5. Pathophysiology of Inflammatory and Autoimmune Diseases in Relation to Gut Dysbiosis
2.5.1. Multiple Sclerosis
Multiple Sclerosis is an autoimmune disease affecting the nervous system, resulting in brain and spinal cord inflammation. Genetic and environmental factors, along with the gut microbiome, play a role in MS. Dysbiosis in gut bacteria can hinder immune function and increase inflammation. Healthy bacteria are crucial for neurotransmitter release, while imbalances can trigger harmful immune responses. Beneficial bacteria produce SCFAs that support the blood-brain barrier and reduce swelling. Research shows that MS patients have fewer beneficial bacteria, such as Bacteroides fragilis and Faecalibacterium prausnitzii, leading to increased inflammation and higher levels of pro-inflammatory bacteria like Akkermansia muciniphila. Gut microbiome disruptions also affect tryptophan metabolism, with MS patients exhibiting lower levels of secondary bile acids, which are vital for reducing inflammation and supporting T cell function (7).
2.6. Microbiota-Gut Axis and Cardiometabolic Diseases
2.6.1. Type 1 Diabetes and Type 2 Diabetes
Research shows significant differences in gut microbiomes between Type 1 diabetes (T1D) and Type 2 diabetes (T2D).
T1D features dysbiosis, with increased harmful bacteria like Bacteroides and Clostridium and decreased beneficial ones such as Lactobacillus and Bifidobacterium. This imbalance can impair immune function, insulin production, gut permeability, and inflammation. In contrast, T2D involves insulin resistance, where the gut microbiome contributes through beneficial metabolites like SCFAs. Butyrate from Firmicutes, like Faecalibacterium prausnitzii, improves insulin response and reduces inflammation. A diverse microbiota, particularly with more Bacteroidetes, is associated with better glucose regulation and lower diabetes risk (8).
2.6.2. Cardiovascular Diseases
Alterations in the gut microbiome are linked to cardiovascular diseases (CVD) such as atherosclerosis and hypertension; gut bacteria affect lipid metabolism, blood pressure, and systemic inflammation. Choline metabolism by gut microbes produces TMAO, a compound that raises cardiovascular risk when levels are high. Dysbiosis can lead to increased TMAO and inflammation, worsening heart health. People with heart disease often have reduced SCFA-producing bacteria, which play a protective role. Polycystic ovary syndrome (PCOS) raises cardiovascular risk with changes in the gut microbiome, inflammation, and hormonal imbalance contributing to increased arterial wall thickness and early signs of atherosclerosis (9).
2.6.3. Obesity and Cardio-Metabolic Health
Obesity is a significant risk factor for cardiometabolic disorders, including cardiovascular disease, type 2 diabetes, hypertension, and chronic kidney disease. Its prevalence has reached pandemic levels, particularly in Western countries, increasing morbidity and mortality (10).
2.6.4. How Gut Microbiota Influence Obesity and Cardiometabolic Risk
The gut microbiome is vital for energy extraction, nutrient uptake, and regulating hormones such as GLP-1, which impacts feelings of fullness and glucose processing. An imbalance in gut bacteria can increase gut permeability, leading to chronic inflammation and metabolic disorders. Changes in gut composition are linked to insulin resistance and metabolic syndrome, with obesity often characterized by reduced microbial diversity and an increase in pro-inflammatory bacteria, which worsens metabolic dysfunction (11, 12).
2.7. Microbiota-Gut Axis and Gastrointestinal Diseases and Cancers
Research indicates that individuals with IBD and IBS have lower gut bacterial diversity compared to healthy individuals, with a decrease in beneficial bacteria from the Firmicutes and Bacteroidetes phyla, such as Lactobacillus and Bifidobacterium. This reduction affects fiber digestion and short-chain fatty acid production, which is crucial for gut health, while harmful bacteria like Proteobacteria may increase, leading to issues like leaky gut and inflammation. In IBD, the loss of Firmicutes disrupts the microbial balance and reduces beneficial species like Faecalibacterium prausnitzii. In IBS-C, characterized by constipation, levels of Methanobrevibacter smithii may rise, worsening the condition (13).
2.8. Gastroesophageal Reflux Disease
Gastroesophageal Reflux Disease (GERD) is a chronic condition where stomach acid irritates the esophagus, leading to heartburn and chest pain. Recent studies suggest that the gut microbiome may influence esophageal motility and inflammation, potentially exacerbating GERD symptoms and impacting immune function (14).
2.8.1. Specific Microbial Changes in Gastroesophageal Reflux Disease
In GERD patients, the Firmicutes to Bacteroidetes ratio alters, impacting gastric acid regulation and motility. A decrease in beneficial Lactobacillus bacteria can lead to increased esophageal inflammation and worsen acid reflux (15).
2.8.2. Gastrointestinal Cancers and the Gut Microbiome
Gut microbiota influences early cancer development through immune responses, inflammation, and DNA repair. While some microbes produce carcinogenic metabolites, others support immune function and epithelial health. Imbalances in gut bacteria can lead to inflammatory responses that damage DNA and activate cancer-promoting pathways, such as NF-κB (16).
2.8.3. Carcinogenic Metabolites
Colorectal cancer is linked to gut bacteria, showing lower levels of beneficial types like Bifidobacteria and Lactobacilli, while harmful bacteria such as Fusobacterium nucleatum and Bacteroides fragilis are elevated. These harmful bacteria can cause inflammation and DNA damage, whereas beneficial compounds like butyrate promote colon health. Gastric cancer is associated with Helicobacter pylori, which contributes to chronic inflammation and gut dysbiosis. Esophageal cancer is connected to harmful bacteria as well, with GERD further disrupting the microbial balance. These findings highlight the need for more research on gut microbiota's impact on cancer for potential treatment and prevention (17).
2.8.4. Microbiota-Gut Axis and Liver Diseases and Cancers
In cirrhosis, chronic liver damage increases intestinal permeability, or "leaky gut," allowing harmful bacteria like E. coli into the bloodstream. This triggers inflammation and immune responses activated by lipopolysaccharides (LPS), leading to more severe liver damage and fibrosis. Cirrhosis alters the gut microbiome, reducing beneficial SCFAs and increasing harmful substances. Bacteria convert dietary components into trimethylamine (TMA), which the liver processes into trimethylamine N-oxide (TMAO), which is linked to liver and heart issues. Additionally, altered bile acids increase fat metabolism and inflammation, further harming the liver (18).
2.8.4.1. Relationship Between Non-fatty Liver Disease, Steatohepatitis, Hepatocellular Carcinoma, and the Gut Microbiota
The gut microbiota significantly influences the progression of non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and hepatocellular carcinoma (HCC) through dysbiosis. In NAFLD, dysbiosis increases intestinal permeability, allowing harmful microbial products like lipopolysaccharides (LPS) to reach the liver, leading to inflammation and lipid accumulation. In NASH, it activates inflammasome pathways and disrupts metabolism, causing liver injury and fibrosis. For HCC, chronic inflammation and dysbiosis facilitate bacterial translocation, promoting carcinogenesis through DNA damage and immune suppression. Overall, gut microbiota dysregulation connects NAFLD, NASH, and HCC, suggesting microbiome modulation as a potential therapeutic target (19).
2.9. Microbiota-Gut Axis and Neurological and Neurodegenerative Disorders
In Alzheimer’s disease (AD), bacteria like Fusobacterium nucleatum increase gut permeability and inflammation, contributing to amyloid beta accumulation. Elevated Bacteroides species are also linked to gut inflammation that negatively impacts brain health and promotes amyloid plaques (20). Parkinson’s disease (PD) is a fast-progressing neurodegenerative disorder marked by the gradual loss of dopamine-producing neurons in the substantia nigra and reduced dopamine activity in the striatum (21). the gut microbiome affects disease progression, as gut inflammation disrupts bacterial balance and causes leaky gut syndrome. This permits harmful substances to enter the bloodstream, leading to brain inflammation and nerve damage. Misfolded alpha-synuclein can reach the brain via the vagus nerve, with Enterococcus faecalis involved. Beneficial bacteria like Prevotella, Lactobacillus, and Bifidobacterium are diminished in PD, reducing protective SCFAs and worsening gut inflammation. Understanding these bacteria is crucial for revealing the mechanisms behind these neurodegenerative diseases (22).
2.10. Techniques and Markers for Assessing the Gut Microbiota
Imbalances in gut microbiota can result in gastrointestinal issues, obesity, insulin resistance, and inflammation. Researchers use methods like 16S rRNA sequencing and metagenomic sequencing to associate specific bacteria with diseases and investigate microbial metabolism. Metabolomics helps identify imbalances, such as elevated lipopolysaccharides in cirrhosis, while FMT shows promise for restoring gut health, especially in Clostridium difficile infections. Inflammatory markers like TNF-α and CRP are significant in conditions like IBD and type 2 diabetes. Flow cytometry tests analyze these markers, and host genetics play a role in microbiome functionality, paving the way for personalized gut health strategies (23).
2.11. Microbiota-Gut Axis and Therapeutic Potential of These
Microbiome-based therapy restores gut bacteria balance to address gastrointestinal issues, mainly through FMT. This method effectively treats recurrent Clostridium difficile infections and may benefit conditions like IBD and IBS (23).
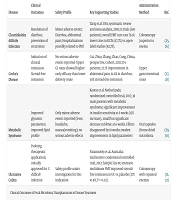
But how FMT Works (24)? Fecal microbiota transplantation is a treatment that helps bring back a healthy balance of gut bacteria, mainly for people with repeated Clostridium difficile infections. It starts with choosing a healthy donor who is tested to make sure they do not have infections, chronic illnesses, or gut problems. Their stool, fresh or frozen, is mixed with saline, filtered, and prepared for use. Fecal microbiota transplantation can be given in several ways: By colonoscopy straight into the colon, by enema, in capsules containing freeze dried bacteria, or through a tube reaching the upper digestive tract. Colonoscopy works best for recurrent Clostridium difficile, while capsules are an easier, non-invasive option. It works by adding beneficial bacteria that displace harmful ones, restoring essential compounds such as SCFAs and antimicrobial peptides, and supporting the immune system to function more normally in non-C conditions. Under different conditions, results may be temporary. The FDA currently approves FMT only for recurrent Clostridium difficile, with 80 - 90% success. Some of these uses are listed in Table 1.
| Disease | Clinical Outcomes | Safety Profile | Key Supporting Studies | FMT Administration Method | Ref. |
|---|---|---|---|---|---|
| Clostridioides difficile Infection | Resolution of diarrhea; prevention of recurrence | Minor adverse events: Diarrhea, abdominal pain; Hospitalizations possibly related to FMT | Tariq et al. USA, systematic review and meta-analysis, 2019; 13 trials (610 patients); overall FMT cure rate 76.1%. lower rates in RCTs (67.7%) vs. open-label studies (82.7%). | Colonoscopy (superior to enema | (25, 26) |
| Crohn's Disease | Induction of clinical remission- Steroid-free remission | No serious adverse events reported- Upper GI route showed higher early efficacy than lower delivery route | Cui, Zhou, Zhang, Zhao, Cong, China, prospective, Cohort, 2021; 174 patients; 72.7% improvement in abdominal pain; 61.6% in diarrhea; 50% steroid-free remission | Upper gastrointestinal route | (27, 28) |
| Metabolic Syndrome | Improved glycemic parameters; improved lipid profile | Only minor adverse events reported (fever, headache, nausea/vomiting); no serious adverse effects | Kootte et al. Netherlands; randomized controlled trial, 2017; 38 male patients with metabolic syndrome; significant improvement in insulin sensitivity at 6 weeks (12% increase).; small but significant decrease in HbA1c at 6 weeks. Effects disappeared by 18 weeks; modest improvements in lipid parameters | Oral capsules (freeze-dried microbiota | (29, 30) |
| Ulcerative Colitis | Evolving therapeutic application; initially approved for C. difficile infection | Safety profile under investigation for this indication | Paramsothy et al. Australia; multicentre randomized controlled trial, 2017; Sample Size 81; intensive multidonor FMT improved steroid-free remission in UC vs. placebo (27% vs 8%, P = 0.02). | Colonoscopy with repeated enemas | (31, 32) |
Abbreviations: FMT, fecal microbiota transplantation.
Microbiome therapies, including probiotics, prebiotics, and synbiotics, are gaining attention for treating digestive conditions. Probiotics such as Lactobacillus rhamnosus and Bifidobacterium longum may reduce inflammation in IBD and IBS, while prebiotics like inulin supports the growth of beneficial bacteria. Synbiotics enhance gut microbiome diversity. Additionally, biological drugs like infliximab and adalimumab target the immune system to reduce IBD inflammation. Alternatives such as ustekinumab and vedolizumab are available for those unresponsive to anti-TNF therapies, and small molecule inhibitors like tofacitinib and ozanimod help control immune responses. Advancements in multi-omics technologies are improving biomarker identification for targeted treatments. While current IBS and IBD therapies are effective, side effects remain a concern, highlighting the need for new strategies such as probiotics, FMT, and phage therapy for better management (Table 2) (33).
| Technology | Mechanism | Potential Applications | Advantages | Key Supporting Studies | Ref. |
|---|---|---|---|---|---|
| CRISPR-loaded virus | Gene editing of bacterial genomes within the gut | Precise modification of specific bacterial populations; potential treatment for microbiome-related diseases | High precision; can target bacteria already established in gut; potential for long-term effects | Lam et al. (2021, 2025), USA; animal study (mouse proof-of-concept); M13 phage delivering CRISPR-Cas9 to E. coli; Stable gene editing of gut bacteria via phage CRISPR in mice, altering E. coli and microbiome composition. | (34) |
| Phage therapy | Bacteriophages target and kill specific bacteria | Alternative to antibiotics for bacterial infections; treatment of antibiotic-resistant infections | High host specificity; Bactericidal effect; self-propagating at infection site; Low toxicity | Wortelboer et al. (2023), Netherlands; double-blind randomized controlled trial (FFT - phage-rich); 24 healthy adults; gut phage administration; administered gut phages; transient alteration of recipient phageome and gut microbiota. Well-tolerated, safe. | (35, 36) |
| Nano synergistic therapy | Synergy between gut microbiota and nanotechnology | Colorectal cancer CRC treatment; enhanced delivery of therapeutic agents | Targeted delivery to specific gut regions; improved bioavailability of therapeutics | Han et al. South Korea, China; Animal interventional study, Mouse (E. coli-induced dysbiosis); Synbiotics with nanoprebiotics (phthalyl pullulan nanoparticles); Synbiotics using nanoprebiotics (phthalyl pullulan nanoparticles) significantly suppressed E. coli, restored gut barrier, increased microbial diversity and beneficial taxa. | (37, 38) |
| Engineered microbiome-modulating approaches | Novel engineering strategies for gut microbiota modulation | Ameliorating side effects of cancer treatments; enhancing efficacy of immunotherapy and chemotherapy | Overcomes gastrointestinal barriers; enhances target-specific delivery; improves drug bioavailability | Arnold et al. (2023), USA; Review & Preclinical research; synthetic biology tools for native gut bacteria; developed gene circuits for E. coli and Bacteroides, modulating microbiome metabolites and immune response. | (39, 40) |
2.12. Diet Influences the Gut Microbiome
Dietary factors significantly impact the gut microbiome, which is essential for immune function and metabolism (Table 3). Fiber, particularly indigestible carbohydrates, promotes the production of beneficial SCFAs, while high-fat and high-sugar diets harm beneficial bacteria. Proteins, including both plant and animal sources, also affect gut health. Polyphenols from fruits, vegetables, and teas support beneficial bacteria and inhibit pathogens. Mediterranean diets, low-FODMAP diets, and probiotics improve gut health, although individual responses vary based on gut bacteria and genetics (41).
| Intervention | Effects on Gut Microbiota | Clinical Outcomes | Key Supporting Studies | Ref. |
|---|---|---|---|---|
| Weight loss | Increased α-diversity; Reduced intestinal permeability; Increased abundance of Akkermansia | Each kg of weight loss associated with increase in α-diversity; reduction in intestinal permeability | Koutoukidis et al. UK, 2022; Systematic Review & Meta-Analysis; 1,916 participants (47 trials); each kg weight loss = 0.012 ↑ α-diversity; ↑ Akkermansia abundance | (42) |
| Mediterranean diet | Increased microbial diversity; higher percentage of SCFAs and fiber-degrading bacteria; increased butyrate-producing bacteria | Reduced TMAO concentration in urine; - potentially reduced incidence of CRC; Improved cardio-metabolic health | Khavandegar et al. Iran, 2024; Systematic Review; 37 studies; MD adherence linked to clinical improvements (glycemia, inflammation, reduced fat mass) | (43, 44) |
| Ketogenic diet | Altered proportions of Actinobacteria, Bacteroidetes, and Firmicutes; significant changes in 19 bacterial genera; decrease in Bifidobacteria | Potential anti-inflammatory effects via ketone body influence on gut microbiome; shifted microbiome correlates with ketone body production | Ang et al. Switzerland, 2025; randomized Controlled Trial;76 overweight/obese individuals; a very low-calorie ketogenic diet improved gut microbial diversity and increased Akkermansia. | (45) |
| Low-carbohydrate diet | Reduced fiber-consuming bacteria; Decreased beneficial bacteria that produce SCFAs | Potential digestive discomfort (constipation, nausea); disruption in gut microbiome diversity | Li et al. China, 2024; Interventional (before–after); Sample Size 43; 79% of participants lost > 5% weight after a 4-week low-calorie diet | (46, 47) |
| Semaglutide | Restored beneficial flora (Akkermansia, Faecalibaculum, Allobaculum); suppressed excessive bacterial abundance; negatively correlated with obesity indicators | Increased tight junction proteins; repaired intestinal barrier function; reduced body weight gain and improved glucose metabolism | Duan et al. China, 2024; Animal study (HFD-induced obese mice); 4 experimental groups; Semaglutide reduced weight gain, improved insulin sensitivity, and increased Akkermansia, which inversely correlated with obesity. | (48) |
| Probiotics | Increased beneficial bacteria abundance; regulated gut microbiota composition | Boosted immune function; improved intestinal barrier integrity; ameliorated symptoms of multiple diseases | Song et al. Korea; Randomized Controlled Trial; obese adults; B. breve + L. plantarum (12-week administration); no significant change in alpha/beta diversity; improved metabolic markers | (49, 50) |
3. Discussion
This review highlights the pivotal role of the gut microbiome in modulating inflammation, immunity, and multi-organ diseases. Key findings demonstrate that dysbiosis contributes to conditions ranging from IBD and IBS to cardiovascular and neurodegenerative disorders, primarily through:
1. Microbial metabolites: The balance between protective SCFAs and harmful TMAO/LPS dictates disease progression.
2. Barrier disruption: Increased intestinal permeability ("leaky gut") drives systemic inflammation.
3. Therapeutic potential: FMT and probiotics show promise, but efficacy varies by disease (e.g., > 80% success in C. difficile vs. 50 - 70% in IBD).
Causality remains unclear in human studies, and microbiome heterogeneity complicates universal treatments. Personalized approaches (e.g., microbiome profiling) and advanced therapies (e.g., phage-CRISPR) may bridge the gap between research and clinical application.
3.1. Conclusions
The gut microbiome is crucial for digestive health and can influence diseases such as IBD, IBS, GERD, and liver cirrhosis. Dysbiosis can cause inflammation and impact immunity, metabolism, and cardiovascular health. Treatments like FMT and probiotics, along with 16S rRNA sequencing, offer potential solutions, with future research needed on causal mechanisms and dietary interventions.