1. Context
Rheumatoid arthritis (RA) is a chronic autoimmune disorder affecting approximately 0.5 - 1% of the global population, with a prevalence of 1 - 2% in women aged 40 - 60 years (1). The RA remains a significant global health challenge. From 1990 to 2019, the age-standardized prevalence rate (ASPR) increased by 0.37%, and the age-standardized incidence rate (ASIR) increased by 0.3%. In 2020, approximately 17.6 million people worldwide were affected by RA, equating to 208.8 cases per 100,000 individuals. Predictions suggest that by 2050, the number of affected individuals will rise to 31.7 million. These trends highlight the need for targeted interventions and resource allocation in regions with a high burden of disease (2).
The RA, characterized by synovial inflammation, leads to cartilage and bone destruction, significantly impairing quality of life and increasing mortality risk by 1.5 - 2-fold compared to the general population (3). Clinically, RA manifests through symmetrical joint involvement, predominantly affecting the small joints of the hands and feet, including the wrists, fingers, and toes (4). The global economic burden is substantial, with annual costs estimated at $20 - 30 billion, including $10 billion in direct healthcare costs and $15 - 20 billion in productivity losses due to disability (5).
Systemic complications of RA, such as cardiovascular disease and osteoporosis, further exacerbate its impact, contributing to a 5 - 10-year reduction in life expectancy (6). In low- and middle-income countries, access to advanced therapies like biologics is limited, with only 10 - 20% of patients able to afford treatments (7) costing $20,000–$50,000 annually, exacerbating health disparities (5).
Conventional RA treatments — non-steroidal anti-inflammatory drugs (NSAIDs), disease-modifying anti-rheumatic drugs (DMARDs), and biologics — face significant limitations, including poor bioavailability, systemic toxicity, and high costs (6). For instance, methotrexate, the cornerstone DMARD, provides clinical benefit in just 30 - 40% of patients and is associated with hepatic toxicity in 10 - 0% (8). Biologics like anti-tumor necrosis factor-alpha (TNF-α) agents are more effective, with response rates of 60 - 70% of cases, but increase infection risks and are cost-prohibitive (6).
Nanotechnology has emerged as a transformative approach to address these challenges, enabling targeted drug delivery, enhanced pharmacokinetics, and reduced side effects (7). Nanoformulations, including nanoparticles, liposomes, micelles, and hydrogels, are designed to target the distinct microenvironment of inflamed joints, which is characterized by leaky vasculature, a mildly acidic pH (~6.0), and elevated reactive oxygen species (ROS), to improve therapeutic efficacy (9). For example, nanoparticles leverage the enhanced permeability and retention (EPR) effect to accumulate in inflamed synovium, while stimuli-responsive systems release drugs in response to specific triggers, achieving a reduction in inflammation in preclinical models (10).
Beyond drug delivery, nanotechnology facilitates biomaterial-based immunotherapies that modulate immune responses, targeting innate and adaptive immune cells to restore immune balance (7). Recent advances have demonstrated the potential of nanotechnology to deliver small interfering RNAs (siRNAs), gene-editing tools, and biologics with high precision, paving the way for personalized RA therapies (11).
This review synthesizes key studies published between 2018 and 2025, focusing on the mechanisms, therapeutic efficacy, and limitations of nanotechnology-based RA therapies. It highlights critical challenges in the field, including scalability, regulatory hurdles, and clinical translation, and proposes future directions to support the development of cost-effective and accessible therapies. Nanotechnology’s potential to transform RA management extends beyond high-income settings, offering scalable solutions to reduce the global burden of autoimmune diseases. Collaborative efforts among researchers, clinicians, and policymakers are essential to translate these innovations into clinical practice, ensuring equitable access to advanced therapies worldwide.
1.1. Pathogenesis of Rheumatoid Arthritis
The RA pathogenesis involves a complex interplay of genetic, epigenetic, environmental, and immunological factors (6). Genetic predispositions, such as HLA-DRB1 alleles, increase RA risk by 2 - 3-fold, while environmental triggers like smoking and infections initiate immune dysregulation (7). The RA is influenced by epigenetic factors. DNA methylation, particularly in CpG islands, regulates gene expression, with hypomethylation in synovial fibroblasts and peripheral blood mononuclear cells (PBMCs) contributing to inflammation and joint damage. Distinct DNA methylation patterns in T cells are associated with altered immune responses and disease progression. Additionally, histone modifications affect chromatin structure, influencing gene accessibility related to inflammation. DNA methyltransferases (DNMTs), especially DNMT1, are essential for maintaining methylation patterns, with a negative correlation observed between DNMT1 and secreted frizzled-related protein 2 (SFRP2) in RA. Non-coding RNAs (ncRNAs) also modulate gene expression and inflammatory processes. Environmental factors, such as diet and stress, can induce epigenetic changes that exacerbate RA. Notably, the reversibility of epigenetic modifications offers promising therapeutic targets for RA management (12).
Innate immune cells, including macrophages and neutrophils, release pro-inflammatory cytokines [TNF-α, interleukin-1 (IL-1), interleukin-6 (IL-6)] that amplify inflammation, promote synovial hyperplasia, and recruit additional immune cells (7). The NF-κB signaling pathway, activated in 80% of RA synovium, drives cytokine production, while the Janus kinase (JAK)/signal transducers and activator of transcription (STAT) pathway mediates IL-6 signaling, contributing to chronic inflammation (11).
Adaptive immune responses, mediated by T and B cells, produce autoantibodies, including anti-citrullinated protein antibodies (ACPAs) and rheumatoid factor (RF), forming immune complexes that exacerbate synovial damage (13). Approximately 70% of RA patients are ACPA-positive, correlating with severe disease (13).
The inflamed synovium exhibits leaky vasculature, hypoxia, and acidic pH (~6.0 vs. 7.4 in healthy tissues), creating opportunities for nanotherapeutics (7). Nanoparticles exploit the EPR effect to accumulate in inflamed joints, achieving 2 - 3-fold higher concentrations than in healthy tissues (10). Stimuli-responsive systems release drugs in response to low pH, high ROS, or matrix metalloproteinases (MMPs), enhancing precision (10).
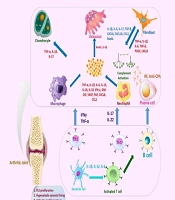
The receptor activator of nuclear factor kappa-Β ligand (RANKL)/RANK pathway drives osteoclastogenesis, leading to bone erosion in 80% of untreated patients within 2 years (13). Fibroblast-like synoviocytes (FLSs) contribute to pannus formation and matrix degradation through MMP-1 and MMP-3, with expression levels 5 - 10 times higher in RA synovium (Figure 1) (14). Recent studies highlight the role of microRNAs (e.g., miR-146a, miR-155) in regulating inflammation, with miR-146a suppressing NF-κB activity in preclinical models (15).
The role of key cells and inflammatory molecules in the pathogenesis of rheumatoid arthritis (RA). The image provides a comprehensive overview of the key cellular and molecular players involved in the pathogenesis of RA, illustrating the complex interactions between different cell types and various inflammatory mediators that contribute to the development and progression of the disease. Chondrocytes, the cartilage-producing cells, are affected in RA, leading to articular cartilage injury. Osteoclasts, responsible for bone resorption, drive bone destruction in RA, influenced by factors like receptor activator of nuclear factor kappa-Β ligand (RANKL), interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and TGF-β. Fibroblasts secrete inflammatory cytokines and chemokines, such as tumor necrosis factor-alpha (TNF-α), IL-1β, and chemokine (C-X-C Motif) ligand 8 (CXCL8), contributing to the inflammatory environment. Activated macrophages release pro-inflammatory mediators, including TNF-α, IL-1β, IL-6, and IL-15, fueling the inflammatory cascade. Neutrophils, recruited to the inflamed joint, release factors like TNF-α, IL-1β, and CXCL8, exacerbating the inflammatory response. Plasma cells contribute to the autoimmune component of RA by producing rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPAs). Dendritic cells, as antigen-presenting cells, play a crucial role in activating T cells, leading to the differentiation of T helper 1 (Th1) and 17 (Th17) cells, which release inflammatory cytokines like interferon gamma (IFN-γ), TNF-α, IL-17, and IL-22. Activated T cells, particularly Th1 and Th17 cells, perpetuate the inflammatory response in RA. B cells are involved in the autoimmune aspects of RA, producing autoantibodies and contributing to the overall inflammatory environment. Additionally, the key pathological features of RA, such as fibroblast-like synoviocyte (FLS) proliferation, hyperplastic synovial lining, articular cartilage injury, bone destruction, reduced joint space, swollen inflamed synovial membrane, and angiogenesis, are highlighted.
Epigenetic modifications, such as DNA methylation of pro-inflammatory cytokine genes (TNF-α, IL-6, and IL-1β), further influence RA progression, suggesting targets for nanotechnology-based gene regulation and therapeutic intervention (11). Understanding these molecular and cellular mechanisms is critical for designing targeted interventions, with nanotechnology offering a promising platform to deliver drugs, siRNAs, or CRISPR/Cas9 tools to specific cellular targets.
1.2. Pharmacological Management of Rheumatoid Arthritis
The RA treatment relies on pharmacological agents, including NSAIDs, conventional and biologic DMARDs, glucocorticoids, and emerging RNA interference (RNAi) therapies (5-8). Despite widespread use, these treatments face significant limitations.
1. The NSAIDs (e.g., ibuprofen, naproxen): Provide symptomatic relief by reducing pain and inflammation but fail to modify disease progression. Long-term use correlates with gastrointestinal bleeding and elevated cardiovascular risks, including myocardial infarction (7).
2. Conventional DMARDs (e.g., methotrexate, leflunomide, sulfasalazine): Methotrexate slows joint damage and suppresses inflammation but causes hepatic toxicity and bone marrow suppression, leading to 30% treatment discontinuation within one year (8). Combination regimens (e.g., methotrexate + leflunomide) enhance efficacy but increase toxicity, with 15 - 20% of patients experiencing severe adverse events (6). Low oral bioavailability (20 - 30%) necessitates high doses, exacerbating toxicity and reducing adherence; 40% of patients show poor compliance (5, 6).
3. Biologics [e.g., TNF-α inhibitors (etanercept), IL-6R blockers (tocilizumab)]: Effective in 60 - 70% of patients but incur prohibitive costs ($20,000 - 50,000 annually), limiting accessibility (only 10 - 20% treated in low-resource settings) (5, 6). Safety concerns include a 2 - 3-fold higher infection risk and tuberculosis reactivation (1 - 2% incidence) (6).
4. The RNAi therapies: Enable precise cytokine targeting (e.g., IL-6, TNF-α) but suffer rapid serum degradation (< 1-hour half-life), poor cellular uptake, and off-target effects, resulting in < 5% clinical success (8). These limitations underscore the need for advanced drug-delivery platforms. Nanotechnology-based systems address these challenges through four key mechanisms:
1. Enhanced bioavailability: Protects therapeutic molecules from metabolic degradation, improving solubility and absorption.
2. Targeted delivery: Uses ligands (e.g., integrin-binding peptides) for site-specific accumulation in inflamed synovium.
3. Reduced toxicity: Minimizes systemic exposure via controlled release, lowering dosing frequency and adverse effects.
4. Cost efficiency: Cuts treatment expenses by 20 - 30% in high-income countries through optimized drug utilization (7).
These advancements improve patient quality of life while reducing hospitalization rates and surgical interventions, alleviating healthcare burdens.
1.3. Nanotechnology-Based Solutions for Rheumatoid Arthritis
Nanotechnology addresses the limitations of conventional RA therapies by improving drug solubility, bioavailability, and targeting. Among these, nanoparticles (20 - 200 nm) stand out for their enhanced solubility and targeted delivery capabilities, though they face challenges such as potential toxicity and clearance by the mononuclear phagocyte system (MPS) (7). Liposomes (50 - 200 nm) offer excellent biocompatibility and drug encapsulation but are limited by stability concerns (1). Micelles and nanoemulsions provide high drug loading and improved solubility, respectively, yet may be limited by rapid clearance or complex formulation requirements. Nanocrystals and solid lipid nanoparticles (SLNs) further enhance bioavailability and biocompatibility but face limitations like aggregation risk or low drug loading capacity (16). Hydrogels, while variable in size, are advantageous for their sustained release and biocompatibility, especially in intra-articular applications, though they require complex fabrication (9). These nanoformulations represent a diverse toolkit for tailoring RA treatment strategies toward more effective, targeted, and patient-specific therapies.
1.3.1. Targeted Drug Delivery Systems
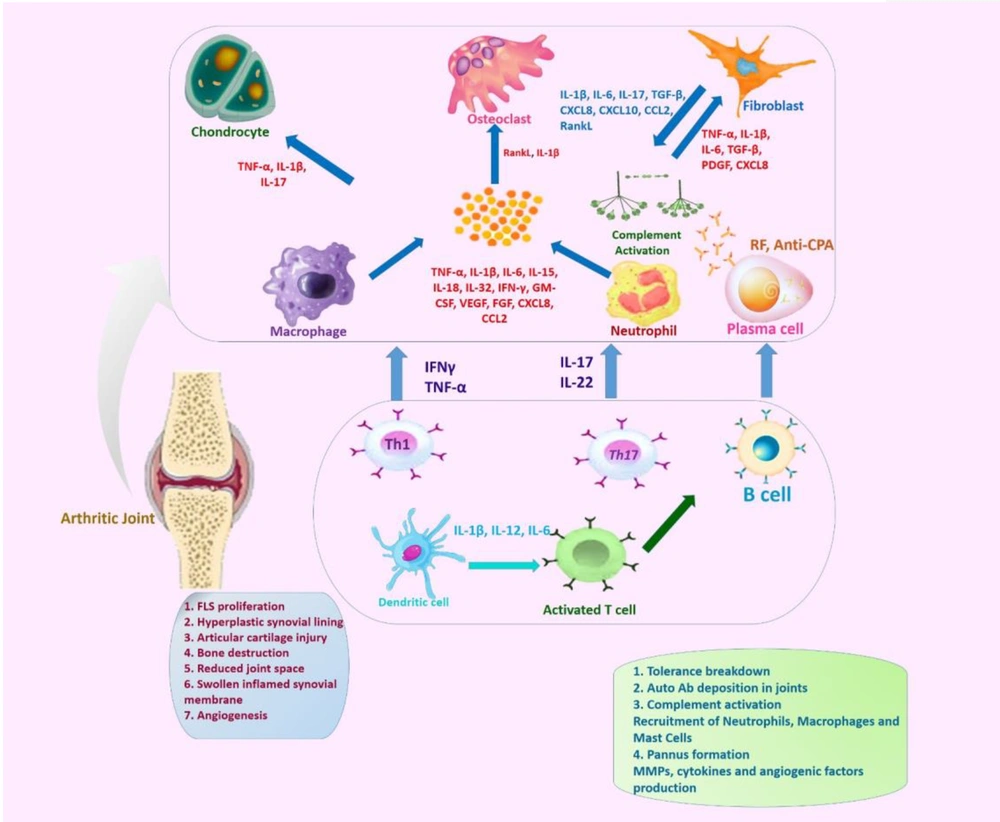
Targeted delivery systems enhance RA therapy by directing drugs to inflamed tissues, reducing systemic toxicity. These systems are categorized into passive, active, and stimulus-responsive strategies (Figure 2).
Targeted therapeutic approaches for rheumatoid arthritis (RA). This image outlines the targeted drug delivery systems and biomaterial-based immunotherapies being explored for RA treatment. Targeted drug delivery: Passive targeting, active targeting, stimuli-responsive, scaffolds, and hydrogels; biomaterial-based immunotherapies.
1.3.1.1. Passive Targeting
Passive targeting leverages the EPR effect, allowing nanoparticles to accumulate in inflamed joints due to leaky vasculature (7). Polyethylene glycol (PEG)-modified nanoparticles, such as dexamethasone-loaded liposomes, enhance circulation time and efficacy in collagen-induced arthritis (CIA) models, resulting in reduced inflammation compared to unencapsulated drug formulations (1). Chitosan-modified nanoparticles enhance biocompatibility, as shown by glycol chitosan formulations delivering methotrexate, achieving 2-fold higher joint accumulation (1). Silica nanoparticles, with high drug-loading capacity, deliver anti-inflammatory agents, reducing TNF-α levels by 50% in murine models (17). Selenium nanocrystals exhibit antioxidant properties, alleviating oxidative stress in RA joints (16). The SLNs delivering curcumin enhance stability and reduce inflammation with minimal toxicity (18). However, clearance by the MPS limits efficacy, with 40 - 50% of nanoparticles cleared within hours (19). Biomimetic nanoparticles, such as neutrophil membrane-coated poly(lactic-co-glycolic acid) (PLGA) nanoparticles, reduce immunogenicity and improve targeting, achieving 3-fold higher joint accumulation (19). Previous studies demonstrated PEGylated lipid nanoparticles delivering tofacitinib, reducing synovial inflammation in CIA models (20).
1.3.1.2. Active Targeting
Active targeting uses ligands to bind specific receptors on inflamed cells, enhancing precision. Folate-conjugated nanoparticles target macrophages expressing folate receptor β, reducing pro-inflammatory cytokines (TNF-α, IL-6) in CIA models (21). Hyaluronic acid (HA)-coated nanoparticles target CD44 receptors, decreasing inflammation and cartilage damage (22). Mannose-modified liposomes delivering p-coumaric acid enhance drug retention in joints, reducing osteoclastogenesis compared to dexamethasone (23). Dextran sulfate-modified nanocomposites target scavenger receptor class A on macrophages, improving methotrexate delivery by 3 - 4-fold (24).
1.3.1.3. Stimuli-Responsive Systems
Stimuli-responsive systems release drugs in response to environmental cues, such as low pH (~6.0 in inflamed joints), high ROS, or enzyme activity. The pH-sensitive micelles deliver siRNA at pH 5.0, silencing inflammatory genes like IL-6 (10). Redox-responsive nanoparticles, triggered by glutathione, release drugs in ROS-rich environments, as shown by indomethacin-loaded polyprodrug amphiphiles reducing inflammation (6). Enzyme-responsive liposomes, activated by phospholipases or MMPs, enable precise drug release, minimizing off-target effects (25). The ROS-responsive nanogels delivering superoxide dismutase reduce oxidative stress in RA models (26). Temperature-responsive liposomes releasing methotrexate at 40°C exploit joint hyperthermia, achieving inflammation reduction (27). Dual-responsive (pH/ROS) nanoparticles deliver tofacitinib, reducing cytokine levels (28). These systems enhance therapeutic precision, but clinical translation requires optimization for stability and reproducibility (7).
1.3.2. Biomaterial-Based Immunotherapies
Biomaterials enable precise modulation of immune responses in RA, targeting innate and adaptive immune cells to restore immune balance.
1.3.2.1. Targeting Innate Immune Cells
1.3.2.1.1. Neutrophils
Doxorubicin-conjugated albumin nanoparticles induce neutrophil apoptosis, reducing inflammation in murine models (29). Sialic acid-decorated liposomes delivering dexamethasone palmitate reduce IL-1β and TNF-α levels (30). Neutrophil membrane-coated nanoparticles act as cytokine sinks, reducing synovial inflammation (19). Lipid nanoparticles delivering IL-10 suppress neutrophil activation. The ROS-scavenging nanoparticles reduce neutrophil-driven oxidative damage (31).
1.3.2.1.2. Macrophages
Folate-decorated liposomes co-delivering methotrexate and NF-κB siRNA target inflammatory M1 macrophages, reducing cytokine production. ROS-sensitive nanoparticles promote M2 macrophage polarization, alleviating arthritis (14). Catalase-incorporated liposomes exploit hydrogen peroxide, enhancing methotrexate release by 3-fold. IL-10-loaded nanoparticles repolarize macrophages, reducing joint swelling (14). Macrophage-derived extracellular vesicles deliver anti-inflammatory miRNAs, reducing inflammation by shifting synovial macrophages from M1 to M2 population (32). Dual-targeting nanoparticles (folate and mannose) enhance macrophage specificity, achieving cytokine reduction (33).
1.3.2.1.3. Dendritic Cells
The PLGA microparticles induce tolerogenic dendritic cells (tDCs), promoting regulatory T cell (Treg) expansion and reducing arthritis symptoms. Liposomes delivering NF-κB inhibitors suppress inflammatory arthritis (14). A clinical trial demonstrated that autologous tDCs treated with vitamin D3 reduced RA disease activity scores (DAS28) (34). Peptide-loaded nanoparticles inducing tDC tolerance enhance Treg function (35). Nanoparticle-based vaccines delivering autoantigens induce tDC-mediated tolerance, reducing inflammation (14).
1.3.2.2. Targeting Adaptive Immune Cells
1.3.2.2.1. T Cells
Nanoparticles encapsulating siRNA targeting c-Rel effectively suppress T helper 1 (Th1)/17 (Th17) cytokines [interferon gamma (IFN-γ), IL-17], halting disease progression in CIA models. Liposome-gold nanoparticles delivering coenzyme Q10 inhibit Th17 cell activity, a key contributor to inflammation at the disease site (36). The PLGA nanoparticles recruiting Tregs via CCL22 enhance immune tolerance and mitigate joint inflammation (36). Nanoparticles delivering STAT3 inhibitors reduce T cell infiltration in synovial tissues (37). Lipid-based nanoparticles silencing PD-1 expression promote Treg differentiation, reducing RA severity (38). Nanovaccines delivering T cell-specific epitopes induce antigen-specific tolerance and significantly lower inflammatory responses in preclinical models (39).
1.3.2.2.2. B Cells
The PEGylated PLGA nanoparticles targeting BAFF (B cell-activating factor of the TNF family) reduce autoreactive B cells and anti-collagen antibodies. Bifunctional nanoparticles combining fibrin peptides and HIV-1 peptides deplete citrullinated protein-specific B cells. Liposomes targeting CD22 receptors induce B cell tolerance, restoring immune balance (40). Nanoparticles neutralizing ACPAs reduce autoantibody-driven inflammation (14).
1.3.2.3. Targeting Non-immune Cells
1.3.2.3.1. Osteoclasts
Mannose-coated liposomes delivering p-coumaric acid impair osteoclastogenesis, reducing bone resorption (14). Berberine-loaded mannosylated liposomes silence inflammatory genes via miR-23a, reducing bone erosion. Gold nanoparticles inhibit osteoclast formation through antioxidant effects, achieving a significant reduction. Zoledronate-loaded nanoparticles prevent bone erosion (14). Alendronate-functionalized nanoparticles target RANKL signaling, reducing osteoclast activity (40). Bisphosphonate-loaded nanoparticles inhibit osteoclastogenesis (41).
1.3.2.3.2. Fibroblast-Like Synoviocytes
Photosensitive PLGA nanoparticles induce ROS-mediated FLS death via photodynamic therapy, reducing inflammation (14). The siRNA-loaded nanoparticles silencing MMP-9 in FLSs reduce tissue remodeling (42). Aptamer-functionalized nanoparticles targeting FLSs deliver JAK inhibitors, alleviating synovial hyperplasia (43). Nanoparticle-delivered miR-124 suppresses FLS proliferation (44).
1.3.3. Scaffolds and Hydrogels
Scaffolds and hydrogels provide localized drug delivery and tissue regeneration, addressing RA’s joint-specific pathology. The HA hydrogels encapsulating methotrexate offer sustained release, reducing joint inflammation in CIA models (45). Thermo-responsive hydrogels combining platelet-rich plasma and black phosphorus nanosheets promote osteogenesis, improving bone repair (46). Transdermal hydrogels enhance methotrexate penetration, improving pharmacokinetics and reducing hepatotoxicity (47). Hydrogels delivering DMARDs, such as iguratimod, reduce cytokine production and promote bone regeneration (48). Infliximab-loaded hydrogels alleviate joint inflammation with minimal systemic exposure (49). Nitric oxide (NO)-scavenging hydrogels modulate cytokine levels, reducing inflammation (50). Smart hydrogels responding to ROS or pH enable on-demand drug release, reducing inflammation (14). Self-healing hydrogels maintain structural integrity under joint stress, enhancing drug delivery (51). 3D-printed scaffolds seeded with mesenchymal stem cells support cartilage regeneration, restoring cartilage thickness (49). Nanofiber scaffolds delivering anti-TNF-α antibodies reduce inflammation and promote tissue repair (52). Nanocomposite hydrogels combining nanoparticles and biologics achieve inflammation reduction and cartilage repair. These systems enhance treatment efficacy, but complex fabrication and high costs limit scalability (53).
1.4. Clinical Applications of Nanomedicine in Rheumatoid Arthritis
1.4.1. Approved Nanomedicines
Between 2012 and 2024, the approval of nanomedicines for RA has been limited (Table 1), with most advancements remaining in preclinical or clinical trial phases. These formulations utilize nanocarriers such as liposomes and polymeric nanoparticles to enhance bioavailability and target joint tissues, aiming to reduce systemic toxicity and dosing frequency. However, regulatory challenges persist due to stringent safety requirements for new nanomaterials. Additionally, no new nanomedicines have been approved by the Food and Drug Administration (FDA) or European Medicines Agency (EMA) for RA between 2023 and 2025, highlighting significant gaps in translating laboratory innovations into clinical practice (54).
| Nanomedicines | Therapeutic Approaches |
|---|---|
| Abraxane (nanoparticle albuminbound paclitaxel) | Used off-label for autoimmune disorders; utilizing nanoparticles for improved delivery |
| Certolizumab pegol (Cimzia) | PEGylated antibody fragment targeting TNFα, enhancing drug delivery and reducing immunogenicity |
| Methotrexate nanoparticles | Nanoparticle formulation of Methotrexate for targeted drug delivery in RA, improving Therapeutic Index |
| Liposomal prednisolone (Nanocort) | Liposomal formulation for enhanced delivery to inflamed tissues, reducing systemic side effects |
| Tofacitinib citrate (Xeljanz) | Nanotechnology-based modifications to improve bioavailability; oral JAK inhibitor |
| Golimumab (Simponi Aria) | Utilizes nanotechnology-enhanced delivery mechanisms for anti-TNFα therapy |
| Nanoparticle-based hyaluronic acid | Nanocarrier for enhanced joint delivery; used in conjunction with other treatments to reduce symptoms |
| Baricitinib (Olumiant) | JAK inhibitor with enhanced drug delivery formulations through nanotechnology approaches |
| Filgotinib (Jyseleca) | Oral JAK inhibitor employing advanced formulation strategies to improve bioavailability |
| Ozoralizumab (Nanozora) | Trivalent anti-TNFα nanobody, specifically designed for inflammatory diseases like RA |
Abbreviations: PEG, polyethylene glycol; RA, rheumatoid arthritis; JAK, Janus kinase.
1.4.2. Ongoing Clinical Trials
As of December 2024, over 30 active clinical trials are investigating nanomedicine-based RA therapies, spanning phase I-IV and observational studies (54). Key approaches include:
1. Targeted biologics: Nanoparticles functionalized with peptides or antibodies to selectively deliver methotrexate or tofacitinib to synovial tissue.
2. Stimuli-responsive systems: The pH-sensitive nanocarriers releasing drugs in inflamed joints (e.g., dexamethasone-loaded liposomes in phase II).
3. Novel formulations: A groundbreaking phase I trial (NCT04877274) evaluates a cinnamaldehyde-based prodrug nanosystem, leveraging FDA-approved food additives for anti-inflammatory effects with reduced hepatotoxicity.
4. Combination therapies: Co-delivery of DMARDs and siRNA via gold nanoparticles to simultaneously suppress inflammation and joint erosion (phase III).
These trials prioritize patient-centric endpoints (e.g., DAS28 scores and quality-of-life metrics) but face recruitment challenges due to RA heterogeneity and stringent inclusion criteria (54).
1.5. Challenges and Future Directions in Nanotechnology-Enabled Combination Therapy for Rheumatoid Arthritis
1.5.1. Current Challenges
1. Safety concerns: Nanomaterials may induce inflammation or oxidative stress, necessitating thorough toxicity evaluations and a deeper understanding of nanomaterial-biological interactions.
2. Drug-drug interactions (DDIs): Co-delivery systems face challenges due to variability in physicochemical properties (e.g., solubility, molecular size), which can impair therapeutic efficacy and pharmacokinetics.
3. Clinical translation: Inconsistent drug delivery within RA's complex microenvironment, patient diversity, manufacturing scalability issues, and stability limitations hinder effective treatment.
4. Regulatory and market barriers: Reluctance from pharmaceutical companies to invest in costly trials and difficulties in demonstrating superiority over conventional therapies impede commercialization (54).
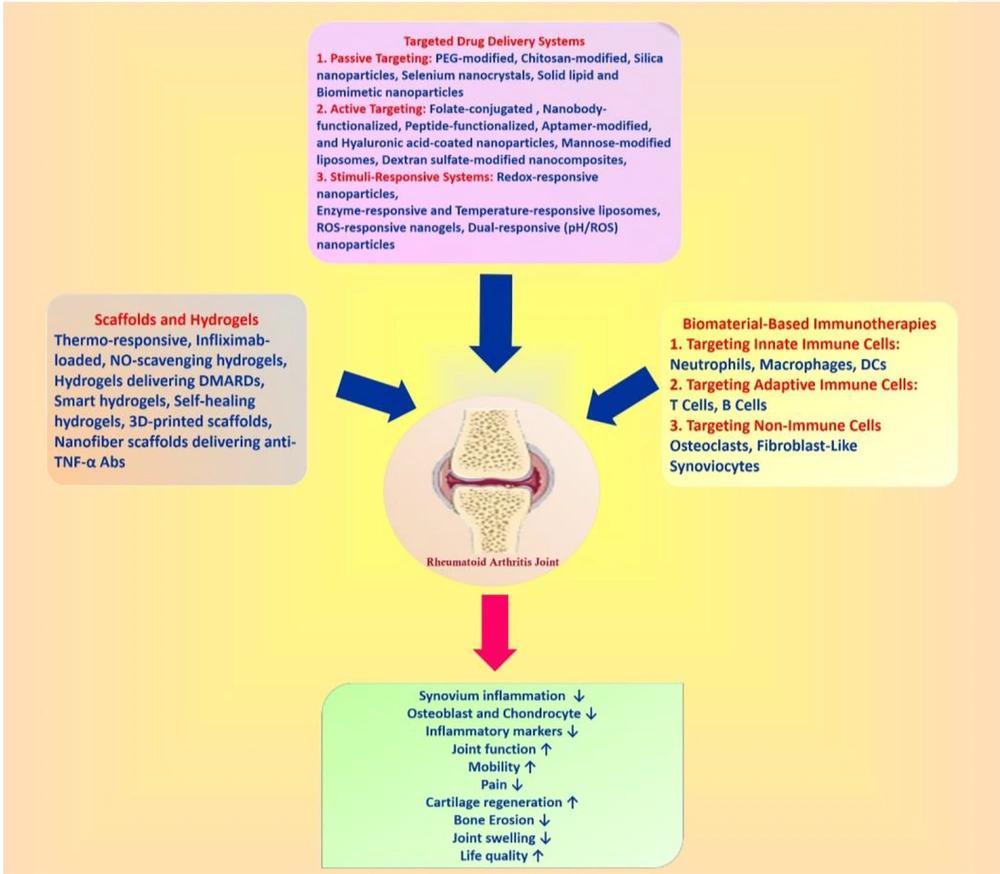
Challenges and limitations of nanotechnology for targeted RA therapy are demonstrated in Figure 3.
Challenges and limitation of nanotechnology for targeted rheumatoid arthritis (RA) therapy. Despite advancements in nanotechnology for combination therapy in RA, several challenges remain (53).
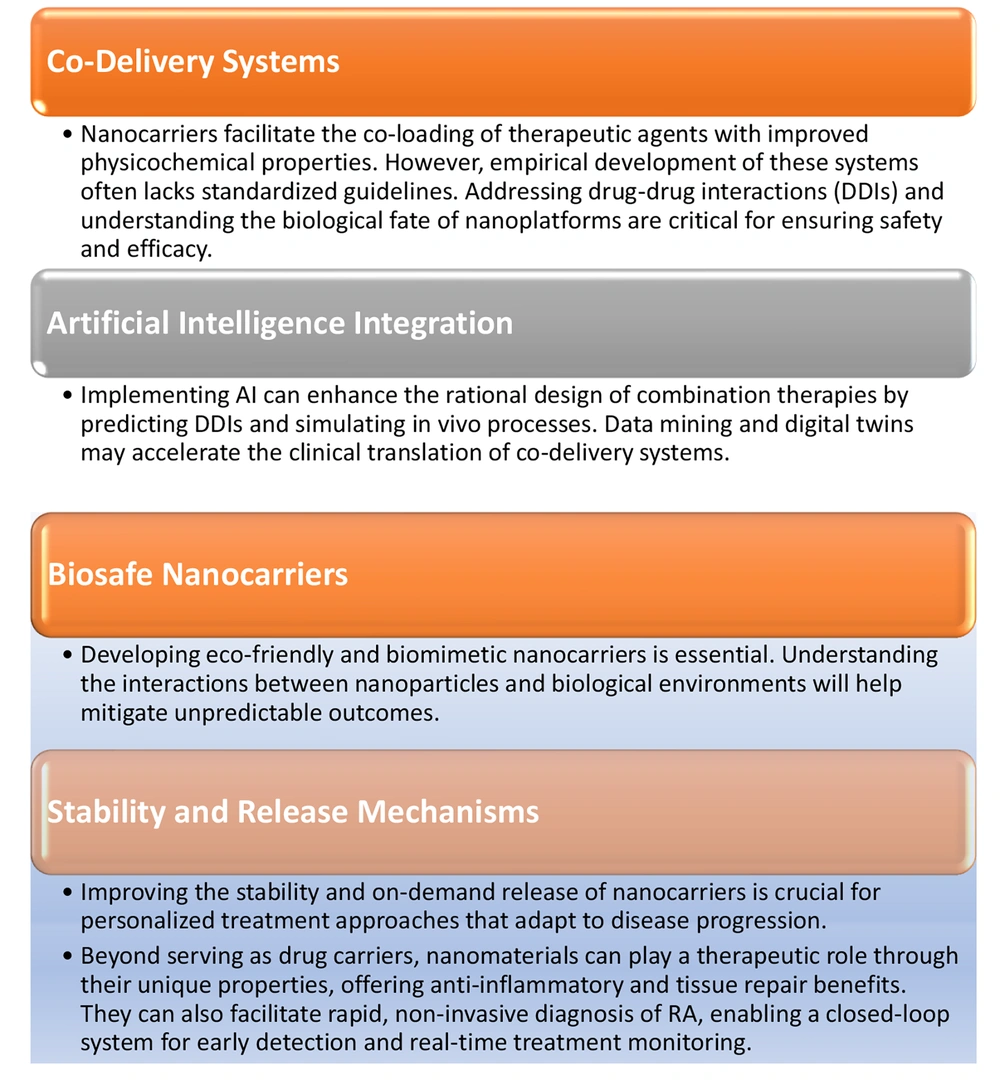
1.5.2. Future Integration Strategies
1. Innovative nanocarriers: Development of biosafe, biomimetic nanocarriers to mitigate safety risks and enhance targeting of joint tissues.
2. Artificial intelligence (AI): Utilizing AI and digital twins to optimize drug design, predict DDIs, and simulate in vivo processes, thereby accelerating clinical translation.
3. Stability and release mechanisms: Enhancing nanocarrier stability and designing stimuli-responsive release mechanisms for personalized treatments adaptable to disease progression.
4. Therapeutic potential of nanomaterials: Exploring the intrinsic anti-inflammatory and tissue-repair properties of nanomaterials, alongside facilitating rapid diagnostics for real-time monitoring (54).
Key strategies for advancing nanotechnology for targeted RA therapy are summarized in Figure 4.
Key strategies for advancing nanotechnology for targeted rheumatoid arthritis (RA) therapy. The RA presents significant challenges in treatment due to its complexity and diversity. While pharmaceutical therapies dominate current clinical practice, nano-empowered combination therapy offers promising solutions to overcome the limitations of monotherapy and associated side effects. This approach leverages synergistic pharmacological mechanisms with minimal overlapping toxicities, enhancing therapeutic efficacy (53).
1.5.3. Addressing Clinical Translation Challenges
1. Heterogeneity and biomarker limitations: Diverse RA subtypes and lack of validated biomarkers complicate trial designs and patient stratification.
2. Long-term safety and drug delivery: Chronic management requires therapies with sustained efficacy, yet biodegradation profiles of nanomaterials remain poorly characterized beyond 24 months.
3. Regulatory hurdles: Extensive safety data requirements delay approvals, and manufacturing scalability issues, along with high production costs, limit accessibility, especially in low-income regions.
4. Patient acceptance: Limited real-world efficacy data and insurance coverage disparities hinder patient acceptance of novel nanotherapies.
To advance nano-empowered combination therapy for RA, it is crucial to implement dynamic, stage-targeted strategies and integrate diagnostics with therapeutics to achieve precision medicine in RA management (53).
2. Conclusions
Nanotechnology represents a transformative frontier in the management of RA, addressing the critical limitations of conventional therapies through innovative drug delivery systems and biomaterial-based immunotherapies. This review highlights the multifaceted role of nanotechnology in enhancing therapeutic efficacy, improving bioavailability, and minimizing systemic toxicity. By leveraging the unique properties of nanoparticles, liposomes, and hydrogels, researchers are developing targeted interventions that can deliver therapeutic agents directly to inflamed tissues, thus maximizing treatment outcomes while reducing adverse effects.
Despite the promising advancements in nanomedicine, several challenges remain in translating these innovations from the laboratory to clinical practice. Safety concerns regarding the biocompatibility of nanomaterials, the complexities of DDIs, and regulatory hurdles pose significant barriers to widespread adoption. Furthermore, the heterogeneity of RA and the absence of validated biomarkers complicate patient stratification and trial design, underscoring the need for tailored approaches to treatment.
Future directions in nanotechnology-enabled RA therapies should focus on the development of biosafe, biomimetic nanocarriers that enhance targeting precision and minimize safety risks. Integrating AI in drug design and utilizing real-time diagnostic tools will further optimize therapeutic strategies, paving the way for personalized medicine in RA management.
In conclusion, the potential of nanotechnology to revolutionize RA therapy is vast, offering scalable solutions that can significantly alleviate the global burden of this debilitating disease. Collaborative efforts among researchers, clinicians, and policymakers are essential to overcome existing challenges, ensuring equitable access to advanced therapies for all patients, regardless of their socioeconomic status. As we continue to explore the intersection of nanotechnology and rheumatology, we move closer to achieving more effective, personalized, and accessible treatments for individuals suffering from RA.