1. Background
Rhinosinusitis (RS) is an important health disorder that reflects an increase in the incidence of allergic rhinitis. The prevalence of RS is various in Iran; for example, the chronic RS prevalence based on the EPOS criteria in Bushehr, a southern region of Iran, was as high as 28.4% (1). Chronic rhinosinusitis (CRS) is defined as an inflammation of the paranasal sinuses (2), which continues for more than 12 weeks with rhinorrhea. Chronic rhinosinusitis consists of two groups, including CRS with nasal polyps (CRSwNP) and without nasal polyps (CRSsNP) (3, 4). The prevalence of allergic rhinitis has been increasing in recent years (5).
The nasal polyp is a multifactorial disease that affects the nose and mucus and is asthmatic in about one-third of the patients. Polyps may develop in other respiratory diseases such as cystic fibrosis, primary ciliary dyskinesia, and immune deficiency. Allergies do not lead to polyps, although it seems that mast cell responses to polyps are important. This explains why corticosteroids are effective in controlling some polyps (6). The formation of polyps is due to epithelial disorders and the migration of the immature branching epithelium (7).
Chronic infection and allergies are known as two major causes of nasal polyp formation. Generally, the initial phase of nasal polyposis may appear with increased exudation of the vessels, lamina propria edema, and nasal mucosa contraction (8). CRSwNP occurs in about 20% of people with CRS. Generally, individuals with CRSwNP have prominent nasal obstruction, hyponymy /anomaly complaints, and less facial pain complaints. These subgroups tend to exhibit resistance to conventional therapy, thus requiring more surgical interventions with higher morbidity. About 80% to 90% of the white patients are diagnosed with CRSwNP and eosinophilia in the tissue (9).
Exhaled NO testing is considered a fast and inexpensive standard diagnostic test for asthma. NO is a vasodilator and inflammatory factor. Additionally, it has an antibacterial effect due to the regulation of blood flow and the frequency of respiratory tract blunt tricks (10, 11). Exposure to NO is a reflection of inflammation in the lower respiratory tract and, thus, lower airways inflammation. Specifically, in patients with eosinophilic inflammation, exhaled NO shows a larger fraction than in healthy controls. High levels of NO are produced in paranasal sinuses through the calcium-dependent NO synthases mechanism, while its normal range is about 25 to 20 ppb (11). On the other hand, NO is also produced by NO induction synthesis (iNOS) in response to inflammation in the nasal mucus (12). NO and its metabolites are toxic to microorganisms and acts as a defense mechanism in the respiratory tract so that it affects the frequency of ciliary beating in the epithelium and tone regulation of the nasal vessels (13). A significant percentage of CRSwNP patients are allergic to environmental allergens (14).
2. Objectives
There is a hypothesis that the exhalation level of NO and sensitivity to aerosols can be related to each other. In this study, we examined this hypothesis to find out the relationship between NO and aeroallergens and determine the relationship between exhaled nitric oxide level and sensitivity to aeroallergens in CRSwNP patients referring to Hazrat Rasool Hospital in Tehran from December 2016 to May 2017.
3. Methods
3.1. Study Design
This study was designed according to a previous study by Green et al. This was a prospective cross-sectional study conducted on patients referring to Hazrat-e-Rasool Akram Hospital in Tehran province from 2016 to 2017. The patients had been diagnosed with CRSwNP according to the EPOS 2012 benchmarks. We evaluated five symptoms in these patients, including nasal congestion, anterior nasal discharge, posterior nasal discharge, facial pain, and decreased olfactory sensation. We used a questionnaire to score the severity of each of these symptoms on a Likert scale (0 - 4 scores).
According to the prick skin test that was positive in 73% of the patients with chronic rhinosinusitis and considering the α error level of 5% and the accuracy of 0.1, the final sample size was computed as 76 patients by WinPepi software (15). Of 89 patients with CRSwNP referring to Hazrat-e-Rasool Akram hospital in Tehran province, 13 patients were excluded from the study due to the lack of a follow-up, and four other patients were excluded due to the defect of their information; thus, the study continued with 72 patients. The inclusion criteria included patients diagnosed with CRSwNP based on the EPOS 2012 criteria. The exclusion criteria included patients with proven immunodeficiency disease.
3.2. Prick Skin Test
The skin prick test, also called the puncture or scratch test, checks for immediate allergic reactions to as many as 50 different substances at once. This test is usually done to identify allergies to pollens, molds, pet dander, dust mites, and foods. In adults, the test is usually done on the forearm. Children may be tested on the upper back. After cleaning the test site with alcohol, the nurse draws small marks on the skin and applies a drop of allergen extract next to each mark. The nurse then uses a lancet to prick the extracts into the skin’s surface. A new lancet is used for each allergen. To see if the skin is reacting normally, two additional substances are scratched into the skin’s surface:
3.2.1. Histamine
In most people, this substance causes a skin response. If you do not react to histamine, your allergy skin test may not reveal an allergy even if you have one.
3.2.2. Glycerin or Saline
In most people, these substances do not cause any reaction. If you do react to glycerin or saline, you may have sensitive skin. Test results will need to be interpreted cautiously to avoid a false allergy diagnosis.
About 15 minutes of skin pricks, the nurse observes the skin for the signs of allergic reactions. If you are allergic to one of the substances tested, you will develop a raised, red, itchy bump (wheal) that may look like a mosquito bite. The nurse will then measure the bump’s size and record the results. Next, the nurse will clean your skin with alcohol to remove the marks. A positive skin test means that you may be allergic to a particular substance. Bigger wheal usually indicates a greater degree of sensitivity. A negative skin test means that you probably are not allergic to a particular allergen.
3.3. Method of FeNO Measurement
Exhaled NO was measured by a NObreath (Bedfont® NObreath®) device in FeNO testing. The Bedfont® NObreath® device is a simple-to-use, battery-powered handheld monitor that measures Fractional exhaled Nitric Oxide (FeNO) in the breath. The device has been designed for use in almost any clinical setting for both children and adults. An adult patient blows into the NObreath® device for 12 seconds, giving an instant result for FeNO in ppb (parts per billion). The higher the FeNO reading, the greater the inflammation in the airways.
3.4. Statistical Analysis
The significance level of 0.05 was considered for all analyses in SPSS v24 software.
4. Results
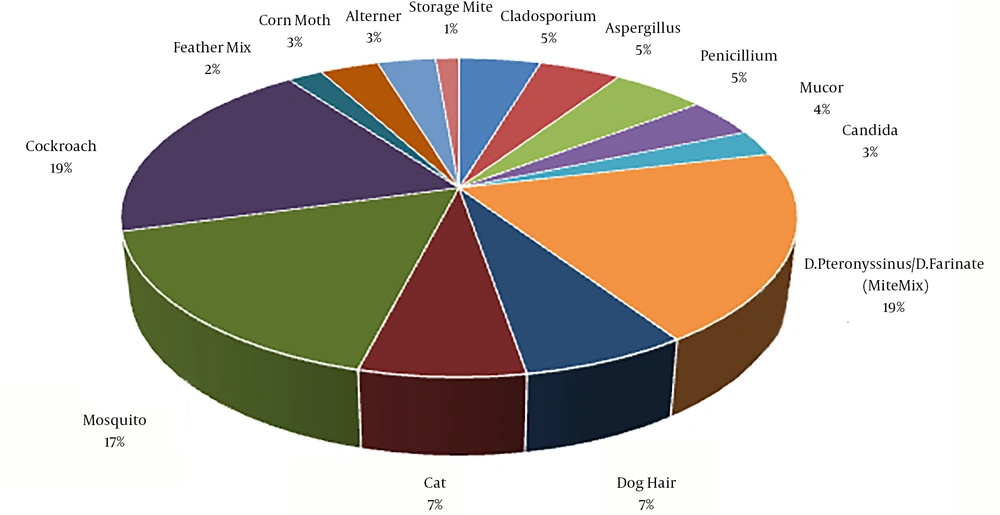
The primary diagnosis of nasal polyps was determined by sinus CT scan in 61 patients, sinus endoscopy in five patients, and both methods in six patients. Besides, 28 patients had a previous history of polyp surgery. The highest level of allergens that were reported positive was related to cockroaches (17.9%) (Figure 1).
On the other hand, the percentage of eosinophils in the nasal smear sample of the participants was 58% (10%), 19% (30-11%), 15% (60-31%), 50% (> 60%) while serum eosinophil levels of the participants were 46% (< 450), 24% (1500-450), and 3% higher than 1500. The correlation of total IgE variables, the absolute number of eosinophils in the blood, and the percentage of eosinophils in the nasal smear with FeNO showed that eosinophils had a significant relationship with FeNO in the nasal smear. In the study of the correlation of FeNO with the sensitivity to indoor aeroallergens, it is first recommended that good fit tests are considered in the estimation of the correlation coefficient with a confidence of 0.95. In other words, if the calculated level is less than 0.05, we will accept the correlation coefficient as an appropriate estimate. However, in some findings, the confidence level of 0.99 means a significance level of 0.001.
According to the results obtained in SPSS V 24, at different levels of significance, the correlation between FeNO and allergens arec mentioned in Table 1. The correlation of the Mosquito and Cockroach were 0.0005 and 0.001, respectively, which were quite significant. and the estimated coefficient is appropriate. Other factors, except for two levels unchanged, showed a weak relationship. However, other factors, except for the two levels that remained unchanged, showed a weak relationship. The lowest correlation was related to the fungi factor with 0.748, and the highest correlation was related to cockroaches with 0.001. Besides, the correlation of FeNO at three levels of normal (< 20 - 25 ppb), elevated (25 - 50 ppb), and high (> 50 ppb) was also tested with three levels of prick testing for aeroallergens (no sensitivity, one positive allergen, two or more positive allergens). less than 57.5% of the patients had more than two positive allergens. The correlation value was estimated to be 0.367 and 0.377. Considering the significance level of 0.001, it can be concluded that there was a significant correlation between FeNO and the triple levels of allergens.
| Fact | Coefficient | Value | Asymptotic Standardized Error | Approximate | Approximate Significance |
|---|---|---|---|---|---|
| Feno*Mold | Unchanged | - | - | - | - |
| Feno*clad | Spearman Correlation | -0.042 | 0.125 | -0.346 | 0.730c |
| Feno*Asper | Spearman Correlation | -0.035 | 0.110 | -0.292 | 0.771c |
| Feno*penicilliums | Spearman Correlation | -0.012 | 0.124 | -0.098 | 0.923c |
| Feno*Mucor | Spearman Correlation | 0.039 | 0.108 | 0.322 | 0.748c |
| Feno*Candid | Spearman Correlation | -0.055 | 0.121 | -0.450 | 0.654c |
| Feno*Danders | Spearman Correlation | 0.213 | 0.119 | 1.798 | 0.077c |
| Feno*Pter | Unchanged | - | - | - | - |
| Feno*Dog | Spearman Correlation | 0.203 | 0.105 | 1.709 | 0.092c |
| Feno*Cat | Spearman Correlation | 0.059 | 0.117 | 0.484 | 0.630c |
| Feno*Mos | Spearman Correlation | 0.293 | 0.115 | 2.523 | 0.014c |
| Feno*Cock | Spearman Correlation | 0.396 | 0.107 | 3.556 | 0.001c |
| Feno*Storage | Spearman Correlation | 0.172 | 0.074 | 1.436 | 0.156c |
| Feno*Feat | Spearman Correlation | -0.009 | 0.134 | -0.076 | 0.940c |
| Feno*Corn | Spearman Correlation | 0.041 | 0.110 | 0.338 | 0.736c |
| Feno*Alter | Spearman Correlation | -0.050 | 0.121 | -0.416 | 0.678c |
5. Discussion
The measurement of FeNO is a quantitative, non-invasive, simple, and safe method for measuring airway inflammation, and is a complementary tool for assessing other respiratory diseases, including asthma (16). In a comparison of arginine isoforms and various types of CRS in 2015, it was reported that exhaled NO is an appropriate marker for the differentiation of CRP phenotypes based on the balance between the activity of arginase and NOS in the production of NO (17).
In a study of 110 patients with asthma in 2014, it was concluded that in patients with mild-to-moderate and severe asthma, blood eosinophils had the highest accuracy in detecting eosinophilic sputum in asthma. The use of blood eosinophils can facilitate individual treatment and management of asthma (18). In a study conducted by Weschta et al. in 2008, they evaluated the performance of a new handheld nitric oxide (NO) analyzer for measuring nasal fractional exhaled respiratory nitric oxide. They concluded that the NIOX MINO Airway monitor (Inflammation Monitor NO) is suitable for measuring nasal FeNO. This may be useful in differentiating hyperplasic eosinophil rhinosinusitis from non-specific CRS. Additionally, nasal FeNO can be used in monitoring the clinical course of CRS with polyps (19). In another study, in 2012, 36 patients with nasal polyposis with eosinophil (ECRS) were treated with either medical or surgical nasal polyps; the evaluation of oral and nasal FeNO levels was done by using an electrochemical NO analyzer in the first and the sixth months. Micro-RNA expression of nitric oxide synthesis (NOS) isoforms in the nasal mucosa and nasal polyposis was analyzed by Polymerase Chain Reaction (PCR) and immunohistochemistry. They concluded that the combination of oral and nasal measurement of FeNO was useful in controlling inflammation in CRS patients. Increasing nasal FeNO levels in the surgical group indicated a rapid improvement in the release of NO from sinus ostia sinus mucus, and the reduction of the oral FeNO level may reflect more severe inflammation of the lower respiratory tract at ECRS (20).
In 1999, Kawamoto et al. conducted a study on two normal and allergic groups exposed to home-made dust. In this study, the expression of iNOS significantly increased in epithelial cells of the allergic group compared to the control group, and this increase in expression happened due to the secretion of pro-inflammatory cytokines (21). In 2005, a study on 100 Thai children with a clinical diagnosis of RS and sinusitis showed that 53% of the patients had a positive prick test for allergens, which confirmed the relationship between the two diseases (22). In 2007, Naraghi et al. reported that the level of NO metabolites in the maxillary sinuses of individuals with chronic sinusitis significantly increased and led to the destruction of the epithelium of the sinuses and could contribute to the pathogenesis of sinusitis (23). In 2010, Guida et al. studied 93 patients diagnosed with CRS, and suggested that the presence of polyps in patients diagnosed with CRS was associated with an increased prevalence of asthma and the exhaled NO level. They also reported that respiratory symptoms without severe bronchospasm, with eosinophilic inflammation of the air tracks, and an increased exhaled NO were related only to patients with nasal polyposis. This is a very important point in distinguishing between the two groups of CRS with or without nasal polyps (24). In 2012, Lee et al. found that the concentration of nasal NO was much higher in patients diagnosed with allergic rhinitis than in normal people, by examining the level of nasal and exhaled NO. Also, in the absence of asthma, allergic rhinitis patients had a higher level of exhaled NO than the normal control group. On the other hand, those with resistant allergic rhinitis had higher exhaled NO concentrations, while there was a lower concentration of nasal NO compared to the control group so that in the severe stages of the disease and rhinitis, which are resistant to NO treatment, nasal NO can be reduced (25). In 2014, Green et al. studied the sensitivity of CRS patients and reported that a high percentage of patients with positive CRS had Positive Prick Test (SPT), and the highest sensitivity in these patients belonged to A. alternata, cat, and Ragweed (15). On the other hand, the role of fungi in CRS has led to a great deal of controversy over CRS. The use of sensitive detection techniques has shown that fungi exist in the intranasal state of all populations, whether patients or healthy, and it can be said that fungi exist in the intranasal of 100% of the population, both CRS and control. This finding suggests that fungi do not significantly correlate with FeNO. However, CRS patients have eosinophils in the nasal and lumen tissues compared to controls and this shows an allergic reaction without increasing IgE levels. These observations suggest a hypothesis: It has been reported that those over-hosting reactions that do not occur through IgE occur as a result of common aerobic fungi that are major causes of the disease in these reactions in most forms of CRS, polyps, and non-polyps. Our findings in this study are parallel to a previous finding (26). In 2013, a comparison of nasal NO level in patients with CRS diagnosis and patients with a diagnosis of colds was done. In this study, the NO concentration was not associated with the symptom score, endoscopic findings, and CT scan, although the nasal NO concentration was not significantly different in cold patients and normal groups. The level of nasal NO was significantly lower in patients diagnosed with CRS than in two other groups (11). In 2015, a study on the comparison of arginine isoforms and various types of CRS subtypes showed that exhaled NO, which was based on the balance between the activity of arginase and NOS in the production of NO, can be an appropriate marker to diagnose various phenotypes of CRS (17).
5.1. Conclusion
This study showed that most people with CRSwNP have a sensitivity to at least one indoor aeroallergen, and cockroaches are the most common allergen in patients with CRSwNP with a prevalence of 17.9%.
The percentage of eosinophils in the nasal mucosa was significantly related to FeNO, and it can be concluded that this index can well predict the FeNO level in these patients. However, the correlation between FeNO and the severity of sinus involvement in CT findings was not significant, and it can be said that CT findings are not an appropriate indicator for the measurement of FeNO, and thus, it is not useful for treatment follow-up and response to treatment in patients.
This study showed that the correlation of FeNO with the sensitivity of these patients to home aeroallergens can be used and this correlation for mosquito and cockroach aeroallergens is very valuable, but for fungi, this correlation had the lowest value of 0.748. Another result of this study is that if nitric oxide levels were considered at three levels of normal (< 20 - 25 ppb), elevated (25 - 50 ppb), and high (> 50 ppb), compared to prick testing of aeroallergens, they could be used to estimate the relapse or lack of appropriate response to medical treatment in patients with CRSwNP. In addition, this study showed that the highest correlation was found between nitric oxide and mosquito and cockroach aeroallergens.
The only limitation of this study was the number of patients.