1. Background
Novel coronavirus disease (COVID-19) is a newly discovered contagious disease caused by the SARS-CoV-2 virus, primarily manifesting as an acute respiratory illness with interstitial and alveolar pneumonia and can affect multiple organs (1). The rapidly spreading outbreak first emerged in Hubei Province in China in December 2019 (2), and subsequently spread to many regions in the world. Iran reported its first confirmed cases of SARS-CoV-2 infections on February 19 2020 in Qom (3). World Health Organization characterizes COVID-19 as a pandemic on March 11 2020 (4). Researchers emphasized that careful surveillance is essential to monitor its future host adaption, viral evolution, infectivity, transmissibility, and pathogenicity (5).
To date (May 1 2020), 3,175,207 confirmed cases of COVID-19 have been reported worldwide and 94,640 in Iran, with 224,172 deaths worldwide (Iran 6,028 deaths) (6). Evidence shows that COVID-19 spreads by human-to-human transmission via droplets, fecal, or direct contact, and has an incubation period estimated at 1 to 14 days (usually 3 to 7 days) (1). This virus infects people of all ages. However, older people and those with underlying medical conditions (such as cardiovascular disease (CVD), diabetes, chronic respiratory disease, and cancer) are at a higher risk of getting severe COVID-19 disease (7). In fact, it seems that people with underlying diseases not only have a higher risk of developing the novel coronavirus (2019-nCoV)-infected pneumonia (NCIP) but also are more likely to die from the virus infection (8). Evidence shows that the majority of infections are mild, presenting with a flu-like illness (1). The most common symptoms of patients with the NCIP were fever (84%), cough (63%), and myalgia and/or fatigue (27%), and mortality was higher in males and elderly patients. Laboratory findings revealed lymphopenia and abnormal C-reactive protein (CRP). Radiological findings mostly described ground-glass opacities and consolidation (9). As well as, evidence shows that symptoms of upper respiratory infection are uncommon (1). A previous study reported that the characteristics of patients outside of Wuhan differed from those initially reported in patients in Wuhan (2). Despite the increasing number of confirmed cases in Iran, the clinical investigation of NCIP patients was insufficient.
2. Objectives
We aimed to describe which one of NCIP patients are at higher risk for severe illness and what is the epidemiological, clinical, laboratory, radiological characteristics and outcomes of the disease
3. Methods
3.1. Design and Setting
In this prospective, case series patients with confirmed NCIP admitted to A and B hospitals from March 1 to March 15, 2020, were enrolled. These hospitals, located in Tehran, Iran, are the major hospitals and are responsible for the treatments for NCIP assigned by the government.
3.2. Data Collection
Data on patients with laboratory-confirmed 2019-nCoV infection by real-time polymerase chain reaction (RT-PCR) and next-generation sequencing were prospectively gathered. The disease outcomes were monitored up to March 18, 2020, the final date of follow-up. Clinical, epidemiological, laboratory, radiological, treatment, and outcomes data were obtained with a questionnaire by two researchers. The questionnaire included demographic data, medical history, signs, symptoms, coexisting conditions, laboratory values, chest computed tomographic (CT) scans, and treatment measures. Data during the hospital stay were collected. The patients were discharged from the hospital once the results of two real-time polymerase chain reaction (Real-time PCR) tests taken 24 hours apart were negative.
3.3. Ethical Consideration
This study was approved by the Ethics Committee of Aja University of Medical Sciences (No. IR.AJAUMS.REC.1398.269) and followed the principles of the Declaration of Helsinki. All patients were informed about the study details and informed consent was obtained from all patients. All information regarding patients was kept confidential, and during all phases of the study, the patients could withdraw their consent to participate without affecting the quality of care provided.
3.4. Statistical Analysis
The statistical analyses were performed using SPSS (Statistical Package for the Social Sciences) version 16.0 software (SPSS Inc). The Kolmogorov-Smirnov test and the Shapiro-Wilk’s W test were used to determine normality. Continuous variables were described using median and interquartile range (IQR) values, and categorical variables were described as frequency rates and percentages. The Mann-Whitney U test was used to compare quantitative variables in both groups (intensive care unit [ICU] care and non-ICU care). Also, χ2 and Fisher’s exact tests were performed for comparing qualitative variables. A P value of less than 0.05 was considered statistically significant.
4. Results
4.1. Patients Characteristics
The study population included 50 hospitalized patients with confirmed NCIP. The median age was 60.0 years (IQR, 41.5 - 68.5; range, 20 - 91 years), 27 (54%) were men (Table 1), and 20 (40%) had blood group O (Table 2). 41 (82%) of patients had used the subway or bus for commuting before the disease and 30 (60%) of them were employed. Of these patients, 33 (66%) were admitted and transferred to the ICU because of the development of illness complications, and 17 (34%) were admitted to isolation wards. The majority of patients had one or more coexisting medical conditions. Hypertension (HTN) (33 [66%]), diabetes mellitus (DM) (29 [58%]), chronic heart failure (19 [38%]), chronic renal failure (19 [38%]), and autoimmune diseases (18 [36%]) were the most common underlying comorbidities. The most common symptoms of illness were fever (50 [100%]), sore throat (50 [100%]), dyspnea (44 [88%]), myalgia (43 [86%]), cough (42 [84%]), fatigue (39 [78%]), and diarrhea (28 [56%]) (Table 1). 12 (24%) patients had a decreased level of consciousness.
| All Patients, No. (%) (N = 50) | ICU Care, No. (%) (N = 33) | No ICU Care, No. (%) (N = 17) | P Valuea | |
|---|---|---|---|---|
| Characteristics | ||||
| Age, median (IQR), y | 60.0 (41.5 - 68.5) | 60.0 (43.0 - 69.0) | 61.0 (34.0 - 68.0) | 0.728 A |
| Age groups | ||||
| 18 - 24 | 3 (6) | 0 (0) | 3 (17.6) | 0.097 B |
| 25 - 49 | 14 (28) | 11 (33.3) | 3 (17.6) | |
| 50 - 64 | 17 (34) | 11 (33.3) | 6 (35.3) | |
| ≥ 65 | 16 (32) | 11 (33.3) | 5 (29.4) | |
| Sex | 0.557 B | |||
| Male | 27 (54) | 19 (57.6) | 8 (47.1) | |
| Female | 23 (46) | 14 (42.4) | 9 (52.9) | |
| Coexisting condition | ||||
| Hypertension | 33 (66) | 26 (78.8) | 7 (41.2) | 0.012 B |
| Diabetes mellitus | 29 (58) | 20 (60.6) | 9 (52.9) | 0.763 B |
| Chronic heart failure | 19 (38) | 13 (39.4) | 6 (35.3) | 1.000 B |
| Chronic renal failure | 19 (38) | 14 (42.4) | 5 (29.4) | 0.540 B |
| Auto immune diseases | 18 (36) | 18 (54.5) | 0 (0) | < 0.001 B |
| Smoker | 27 (54) | 18 (54.5) | 9 (52.9) | 1.000 B |
| Signs and Symptoms | ||||
| Fever | 50 (100) | 33 (100) | 17 (100) | |
| Highest temperature, ºC, median (IQR) | 39.0 (38.5 - 40.0) | 39.0 (38.0 - 39.6) | 38.9 (38.5 - 40.0) | 0.236 A |
| 37.3 - 38.0 | 11 (22) | 9 (27.3) | 2 (11.8) | 0.408 C |
| 38.1 - 39.0 | 19 (38) | 11 (33.3) | 8 (47.1) | |
| > 39.0 | 20 (40) | 13 (39.4) | 7 (41.2) | |
| Cough | 42 (84) | 29 (87.9) | 13 (76.5) | 0.419 B |
| Dyspnea | 44 (88) | 33 (100) | 11 (64.7) | 0.001 B |
| Sore throat | 50 (100) | 33 (100) | 17 (100) | |
| Myalgia | 43 (86) | 32 (97) | 11 (64.7) | 0.004 B |
| Fatigue | 39 (78) | 24 (72.7) | 15 (88.2) | 0.292 B |
| Diarrhea | 28 (56) | 21 (63.6) | 7 (41.2) | 0.147 B |
| Highest respiratory rate, median (IQR) | 32.0 (28.0-34.0) | 31.0 (28.5-33.0) | 32.0 (26.0-35.0) | 0.299 A |
| Respiratory rate > 24 bpm | 48 (96) | 32 (97) | 16 (94.1) | 1.000 B |
| SPO2, median (IQR) | 78.0 (76.0-80.0) | 78.0 (76.0-80.0) | 78.0 (77.0-80.0) | 0.313 A |
Abbreviations: bpm, beats per minute; ICU, intensive care unit; IQR, interquartile range; 2019-nCoV, 2019 novel coronavirus.
aP values comparing ICU care and no ICU care are from AMann-Whitney U test, BFisher’s exact test, or Cχ² test.
| Normal Range | All Patients, Median (IQR) (N = 50) | ICU Care, Median (IQR) (N = 33) | No ICU Care, Median (IQR) (N = 17) | P Valuea | |
|---|---|---|---|---|---|
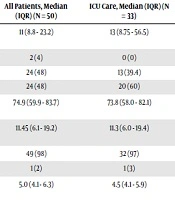
| White blood cell count, × 103/ul | 4 - 11 | 11 (8.8 - 23.2) | 13 (8.75 - 56.5) | 9.4 (8.8 - 11.5) | 0.108 A |
| < 4 | 2 (4) | 0 (0) | 2 (11.8) | 0.015 B | |
| 4 - 11 | 24 (48) | 13 (39.4) | 11 (64.7) | ||
| > 11 | 24 (48) | 20 (60) | 4 (23.5) | ||
| Neutrophil count, × 103/ul | 25 - 75 | 74.9 (59.9 - 83.7) | 73.8 (58.0 - 82.1) | 77.4 (61.6 - 86.6) | 0.525 A |
| Lymphocyte count, × 103/ul (%) | 15 - 35 | 11.45 (6.1 - 19.2) | 11.3 (6.0 - 19.4) | 14.3 (5.9 - 18.8) | 0.870 A |
| < 15 | 49 (98) | 32 (97) | 17 (100) | 0.660 B | |
| ≥ 15 | 1 (2) | 1 (3) | 0 (0) | ||
| Monocyte count, × 103/ul | 2 - 8 | 5.0 (4.1 - 6.3) | 4.5 (4.1 - 5.9) | 5.2 (4.0 - 6.4) | 0.652 A |
| Platelet count, × 103/ul | 150 - 450 | 127.5 (74 - 204) | 130 (77.5 - 204.5) | 120 (58 - 160) | 0.616 A |
| < 150 | 32 (64) | 22 (66.7) | 10 (58.8) | 0.212 B | |
| 150 - 450 | 16 (32) | 11 (33.3) | 5 (29.4) | ||
| > 450 | 2 (4) | 0 (0) | 2 (11.8) | ||
| Alanine aminotransferase, IU/L | 5 - 40 | 50.0 (48.0 - 56.0) | 49.0 (48.0 - 55.0) | 52.0 (49.0 - 62.0) | 0.094 A |
| > 40 | 50 (100) | 33 (100) | 17 (100) | ||
| Aspartate aminotransferase, IU/L | 5 - 40 | 53.5 (48.0 - 56.0) | 54.0 (48.0 - 57.0) | 51.0 (47.5 - 55.5) | 0.417 A |
| 5 - 40 | 2 (4) | 0 (0) | 2 (11.8) | 0.111 B | |
| > 40 | 48 (96) | 33 (100) | 15 (88.2) | ||
| PH | 7.38 - 7.42 | 7.33 (7.31 - 7.36) | 7.33 (7.30 - 7.35) | 7.36 (7.32 - 7.49) | 0.015 A |
| Pco2 | 35 - 45 | 35.0 (32.7 - 45.2) | 35.0 (33.0 - 43.0) | 35.0 (32.0 - 49.0) | 0.734 A |
| Hco3 | 22 - 26 | 19.0 (16.0 - 21.0) | 20.0 (16.0 - 22.0) | 18.0 (16.5 - 19.0) | 0.125 A |
| Vitamin D, ng/mL | 30 - 100 | 6.0 (5.0 - 8.0) | 7.0 (5.0 - 9.0) | 6.0 (5.5 - 7.0) | 0.316 A |
| Deficiency < 10 | 49 (98) | 32 (97) | 17 (100) | 1.000 B | |
| Insufficiency 10 - 29 | 1 (2) | 1 (3) | 0 (0) | ||
| Erythrocyte sedimentation rate, (1 Th Hr), mm/h) | Male < 20; Female < 30 | 35.0 (25.0 - 40.0) | 35.0 (26.5 - 39.5) | 35 (24.0 - 40.0) | 0.689 A |
| C. R. Protein (positive) | 43 (86) | 30 (90.9) | 13 (76.5) | 0.210 B | |
| Lactate dehydrogenase, U/L | 225 - 500 | 692.5 (580.0 - 745.5) | 698.0 (589.0 - 763.5) | 698.0 (530.0 - 721.5) | 0.418 A |
| Creatinine, mg/dL | Male: 0.9 - 1.3; Female: 0.6 - 1.1 | 3.50 (1.4 - 5.0) | 4.8 (1.8 - 5.1) | 2.3 (1.2 - 3.5) | 0.002 A |
| Urea, mg/dL | 10 - 50 | 123.0 (84.0 - 135.0) | 124.0 (83.0 - 134.0) | 98.0 (84.0 - 135.0) | 0.572 A |
| > 50 | 50 (100) | 33 (100) | 17 (100) | ||
| Blood group, f (%) | |||||
| A | 8 (16) | 7 (21.2) | 1 (5.9) | 0.105 C | |
| B | 13 (26) | 8 (24.2) | 5 (29.4) | ||
| AB | 9 (18) | 8 (24.2) | 1 (5.9) | ||
| O | 20 (40) | 10 (30.3) | 10 (58.8) | ||
| Bilateral involvements on chest radiographs (patchy shadows or ground glass opacity) | 31 (62) | 31 (93.9) | 0 (0) | < 0.001 B |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; 2019-nCoV, 2019 novel coronavirus.
aP values comparing ICU care and no ICU care are from AMann-Whitney U test, BFisher’s exact test, or Cχ² test.
Compared with patients who did not receive ICU care, patients who required ICU care were more likely to have some of underlying comorbidities, including HTN (26 [78.8%] vs. 7 [41.2%], P = 0.012), and auto immune diseases (18 [54.5%] vs. 0 [0%], P < 0.001). Compared with the non-ICU patients, patients admitted to the ICU were more likely to report dyspnea (33 [100%] vs. 11 [64.7%], P = 0.001) and myalgia (32 [97%] vs. 11 [64.7%], P = 0.004). Other characteristics, sign and symptoms did not differ between ICU and non-ICU patients (P > 0.05) (Table 1).
4.2. Laboratory Parameters
The majority of patients had lymphopenia 49 (98%), 24 (48%) leukocytosis, and 32 (64%) of them had thrombocytopenia. Other laboratory abnormalities were depressed Hco3 and elevated LDH, erythrocyte sedimentation rate, alanine aminotransferase, aspartate aminotransferase, creatinine, and blood urea. As well as 43 (86%) patients had a high level of CRP. All of the 50 enrolled patients had a deficiency or insufficiency in vitamin D.
There were some differences in laboratory findings between ICU and non-ICU patients, including higher white blood cells (P = 0.015), as well as higher levels of creatinine (P = 0.003) (Table 2). Other parameters did not differ between patients who received ICU care and patients who did not receive ICU care.
All of the patients showed the involvement of a chest CT scan. Of the 50 patients, 31 (62%) had bilateral involvements. The typical findings of chest CT images of patients were ground-glass opacity.
4.3. Common Complication and Treatment
All patients had pneumonia. Common complications among the patients included acute respiratory distress syndrome (ARDS) (18 [36%]), arrhythmia (19 [38%]), and shock (14 [28%]) (Table 3).
| All Patients, No. (%) (N = 50) | ICU Care, No. (%) (N = 33) | No ICU Care, No. (%) (N = 17) | P Valuea | |
|---|---|---|---|---|
| Decreased level of consciousness | 12 (24) | 10 (30.3) | 2 (11.8) | 0.181 |
| Illness complication | ||||
| ARDS | 18 (36) | 9 (27.3) | 9 (52.9) | 0.119 |
| Arrhythmia | 19 (38) | 13 (39.4) | 6 (35.3) | 1.000 |
| Shock | 14 (28) | 8 (24.2) | 6 (35.3) | 0.511 |
| Drug therapy | ||||
| Hydroxychloroquine | 33 (66) | 20 (60.6) | 13 (76.5) | 0.351 |
| Oseltamivir | 35 (70) | 22 (66.7) | 13 (76.5) | 0.533 |
| Lopinavir/ritonavir | 29 (58) | 19 (57.6) | 10 (58.8) | 1.000 |
| Ciprofloxacin | 9 (18) | 5 (15.2) | 4 (23.5) | 0.467 |
| Vancomycin | 19 (38) | 14 (42.4) | 5 (29.4) | 0.540 |
| Levofloxacin | 14 (28) | 10 (30.3) | 4 (23.5) | 0.746 |
| Paracetamol | 30 (60) | 21 (63.6) | 9 (52.9) | 0.548 |
| Diphenhydramine | 35 (70) | 22 (66.7) | 13 (76.5) | 0.533 |
| Oxygen support | ||||
| Noninvasive ventilation | 22 (44) | 14 (42.4) | 8 (47.1) | 0.773 |
| Invasive mechanical ventilation | 17 (34) | 10 (30.3) | 7 (41.2) | 0.534 |
| Extracorporeal membrane oxygenation | 4 (8) | 4 (12.1) | 0 (0) | 0.285 |
| Outcome disease | ||||
| Discharges | 37 (74) | 22 (66.7) | 15 (88.2) | 0.173 |
| Mortality | 13 (26) | 11 (33.3) | 2 (11.8) |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; 2019-nCoV, 2019 novel coronavirus.
aP values comparing ICU care and no ICU care are from Fisher’s exact test.
Most patients received a class of antimalarial drugs (hydroxychloroquine, 33 [66%]), antiviral therapy (oseltamivir, 35 [70%]), antiretroviral therapy (lopinavir and ritonavir, 29 [58%]), antibacterial therapy (ciprofloxacin, 9 [18%]; vancomycin, 19 [38%]; levofloxacin, 14 [28%]), and many received antipyretic therapy (paracetamol, 30 [60%]) and antihistamines (diphenhydramine, 35 [70%]) (Table 3).
Invasive mechanical ventilation was required in 17 (34%) patients and 4 (8%) patients received extracorporeal membrane oxygenation.
As of March 18, 2020, 37 (74%) of 50 patients discharged and 13 (26%) patients died.
5. Discussion
This study reported a prospective, case series of 50 hospitalized patients with laboratory-confirmed 2019-nCoV infection. Patients had serious pneumonia and were admitted to two hospitals in Tehran, Iran. Patients with severe illness admitted to ICU.
In this study, most patients presented with fever, dyspnea, myalgia, cough, and fatigue that indicate lower respiratory tract infection. Some of these features reported in other studies (5, 10). In this study, all patients had at least one sign and symptom of upper respiratory tract infection (e.g., sore throat), indicating that the lower airway was infected. A recent study reported that the majority of patients infected with SARS-CoV-2 in Singapore (61%) had a sore throat (11). In contrast, Huang et al. reported that a few patients had rhinorrhea, sneezing, or sore throat (5). Moreover, in the present study, 56% of patients had diarrhea. Previous studies reported that 3% (5) to 10.1% (10) patients developed intestinal signs and symptoms (e.g., diarrhea). Compared with symptoms in non-ICU patients, some symptoms were more common in critically ill patients, including dyspnea and myalgia. In this regard, Wang et al. found that dyspnea, abdominal pain, and anorexia were more common in ICU patients (10).
In the present study, most hospitalized NCIP patients suffer from chronic illness and patients who had serious underlying chronic illness had a higher risk for severe illness. HTN, DM, chronic heart failure, chronic renal failure, and autoimmune diseases were the most common coexisting conditions. Evidence showed that the most commonly reported coexisting conditions are DM, chronic lung disease, and CVD (12). Richardson et al. reported that the most common comorbidities in patients with COVID-19 hospitalized in a US health care system were hypertension (56.6%), obesity (41.7%), and diabetes (33.8%) (13). In the present study, compared with patients who did not receive ICU care, patients who required ICU care were more likely to have HTN and autoimmune diseases. In this regard, some researchers believed that an over-immune reaction might cause some damage during infections. This reaction has been seen during the outbreak of SARS that may be true of COVID-19 infection (12). Other researchers reported that compared with the non-ICU patients, patients admitted to the ICU were more likely to have HTN, DM, CVD, and cerebrovascular disease (10). These suggest that comorbidity may be a risk factor for poor outcomes.
However, there was no difference in the proportion of men and women between ICU and non-ICU patients. Similar findings reported in recent studies (5, 10). Also, there was no difference in the age of ICU and non-ICU patients. These data are similar to a previous study (5) and differs from the recent report that showed the patients admitted to the ICU were older (10). The most common laboratory abnormalities observed in this study were depressed platelet count, Hco3, lymphocytes, vitamin D, and elevated LDH, erythrocyte sedimentation rate, CRP, alanine aminotransferase, aspartate aminotransferase, creatinine, and blood urea. Similarly, Wang et al. reported that lymphopenia and elevated LDH occurred in 70.3% and 39.9% of patients, respectively (10). Compared with non-ICU patients, patients who received ICU care had higher levels of white blood cell and creatinine. These findings indicate that infection with 2019-nCoV may be associated with cellular immune deficiency, activation of inflammatory factors, hepatic injury, and kidney injury. Similar findings have been reported in other studies (10).
A significant prevalence of vitamin D deficiency among the Iranian population has been reported (14). In this study, all patients had deficiency or insufficiency in vitamin D. Some researchers hypothesize that raising serum 25(OH) D concentrations through vitamin D supplementation could reduce the incidence, severity, and risk of death from influenza, pneumonia, and the current COVID-19 epidemic (15). More studies addressing dosage and combinations of micronutrients such as vitamin D in different populations are required to substantiate the benefits of micronutrient supplementation against infection. In the present study, 62% of the patients had bilateral involvement with ground-glass opacities on chest CT scans. Recent studies reported that 98% (5) to 100% (10) of patients had bilateral involvements. Common complications among these patients included ARDS, arrhythmia, and shock. Similar findings reported in a previous study (10).
So far, no standard treatment has been proven or endorsed currently by the Centers for Disease Control and Prevention or World Health Organization (16). At the time of the study, according to the diagnosis and treatment of COVID-19 flowchart at outpatient and inpatient services levels in Iran NCIP patients who had dyspnoea or pulse oximetry of less than 93 were admitted in hospitals. The triple therapy regimens in hospitalized NCIP patients included oseltamivir, hydroxyquinoline sulfate, and lopinavir/ritonavir. If the patient’s level of consciousness decreased, respiratory rate ≥ 24, blood pressure < 90/60, multilobar infiltration in chest X-ray or CT-scan, and/or hypoxia, ribavirin was added to the mentioned drugs (17). It should be noted that some of these drugs have now been deleted from patients’ treatment protocol. Myocardia injury, arrhythmia, kidney injury, and hepatotoxicity could have been related to the direct effects of the virus, side effects of drug therapy, hypoxia, and shock. Currently, there are drug therapy issues. Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy.
In this study, we found a relatively high mortality rate for COVID-19 of up to 26%, which is higher than in the previous reports. Recent studies reported that the mortality rate of NICP patients was 0% (2), 4.3% (10), 14.1% (18), and 15% (5) but at the time of these reports, many patients were still hospitalized, and their outcome is unclear. This is likely due to a large proportion of severely or critically ill patients admitted to the hospitals under study, that designated hospitals for severe COVID-19 in Tehran, and the medical resource limitations at the beginning of the COVID-19 outbreak in Iran.
This study has some limitations. Firstly, we investigated a limited number of cases. In fact, this is a modest-sized case series of patients admitted to the hospitals. Many patients were continually being admitted to these hospitals as data were being gathered, and thus we obtained data on most but not all of the patients with laboratory-confirmed infection during the study period. Secondly, we enrolled only patients admitted to the hospitals. The mortality rate of hospitalized patients was high. As patients with a higher degree of illness are admitted to the hospital, these patients will have a poorer prognosis than all infected patients. Further studies in outpatient, primary care, or community settings are needed to get a full picture of the clinical characteristics and natural history of 2019-nCoV infection.
5.1. Conclusion
Hospitalized NCIP patients who have serious underlying chronic diseases might be at higher risk for severe disease. Common symptoms of illness were fever, sore throat, dyspnea, myalgia, cough, and fatigue. Major complications during hospitalization included ARDS, arrhythmia, and shock. Bilateral distribution of patchy shadows and ground-glass opacity was a typical hallmark of CT scans for NCIP. The 2019-nCoV infection is now spreading globally. Currently, there is no effective drug treatment. Further research and surveillance are crucial to develop drugs against 2019-nCoV infection as soon as possible. Gaps in our knowledge need fulfillment by future studies with a higher sample size.