1. Background
Breast cancer (BC) is one of the most common cancers among women, especially in these days (1, 2). The incidence of BC is about 24.6% of all cancers that happen in women (3). In the previous decades, although the prevalence of BC-related mortality was high among patients, the survival rate of patients would increase based on the recent diagnosis and treatment strategy (4-6). The main problem in BC treatment is cardiotoxicity derived chemotherapy. BC is a heterogeneous disease so it has a variety of clinical and histological symptoms among the patients, but proper treatment can be helpful in the management of the symptoms (7-9). Anthracyclines such as Adriamycin and danomycin are one of the most commonly used chemotherapy, which inhibit the progressive growth and metastasis (10, 11). Studies indicated that the use of these drugs can affect the cardiovascular system and increase cardiotoxicity. Anthracyclines increase the production of reactive oxygen species (ROS). ROS production impairs cardiomyocyte function and induced cardiotoxicity. Anthracycline also leads to cardiac cell apoptosis due to the production of inflammatory mediators, impaired calcium metabolism, mitochondrial function, and ATP production (12). Several risk factors such as smoking, old age, history of heart disease can increase the possibility of cardiotoxicity among BC patients under chemotherapy (13, 14). Therefore, the detection of risk factors in BC patients and related pathogenesis mechanisms in cardiotoxicity induced by chemotherapy agents can be useful in the management of heart disease in cancer patients. Cho et al. evaluated the incidence of cardiotoxicity in BC patients. They showed risk factors such as old age, metastasis, and concomitant use of doxorubicin and trustuzumab could increase the incidence of cardiotoxicity in cancer patients (15). Chung et al. demonstrated that cardiotoxicity could occur in patients treated with doxorubicin at 300 mg / m dosage (2) and higher (16).
2. Objectives
Several risk factors are known to be associated with the occurrence of cardiotoxicity in patients, finding the main risk factors in patients can be very helpful in designing preventive strategies and identifying high-risk patients. However, these risk factors are no fully identified in Iran. So, it can be an important approach to prevent the occurrence of cardiotoxicity in patients. The present study aimed to consider the relation between the risk factors and induced cardiotoxicity in BC patients hospitalized in Ahvaz Golestan hospital.
3. Methods
3.1. Patients and Control Selection
All the participants in this study (n = 66), confirmed between 2017 - 2018, went to the oncology section of Ahvaz Golestan hospital to receive chemotherapy treatment. Table 1 provides all demography data and clinical parameters related to patients.
| No. (%) | |
|---|---|
| Age | |
| 20 - 30 | 0 (0) |
| 30 - 40 | 9 (13.6) |
| 40 - 50 | 27 (40.9) |
| > 50 | 30 (45.5) |
| History of Smoking | |
| Yes | 1 (1.5) |
| No | 65 (98.5) |
| BMI | |
| ≥ 30 | 19 (28.8) |
| < 30 | 47 (71.2) |
| History of heart disease | |
| Yes | 8 (12.1) |
| No | 58 (87.9) |
| History of Blood pressure | |
| ≥ 135/85 | 16 (24.2) |
| < 135/85 | 50 (75.8) |
| History of Diabetes | |
| FBS ≥ 126 | 14 (21.2) |
| FBS < 126 | 52 (78.8) |
| HDL | |
| ≥ 40 | 55 (83.3) |
| < 40 | 11 (16.7) |
| LDL | |
| ≥ 100 | 18 (27.3) |
| < 100 | 48 (72.7) |
| TG | |
| ≥ 150 | 11 (16.7) |
| < 150 | 55 (83.3) |
| Cholesterol | |
| ≥ 200 | 16 (24.2) |
| < 200 | 50 (75.8) |
| Physical activity | |
| Yes | 8 (12.1) |
| No | 58 (87.9) |
| CRP | |
| ≥ 6 | 5 (7.6) |
| < 6 | 61 (92.4) |
Characteristics of Patients with Breast Cancer
3.2. Inclusion Criteria
Age > 20 years, confirmed BC according to histology and laboratory symptoms, absence of underlying diseases, and availability of the patient’s information.
3.3. Exclusion Criteria
Heart dysfunction in the early treatment stage (first time echo), congenital heart disease, use of drugs that effect on heart function, cancel of follow-up, unavailability of patient information, and underlying disease. The following formula was used to determine the sample size based on reference (17). Before launching the study, informed consent was obtained from each patient. This study was approved by the ethics committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1397.053).
Sample size determination formula:
3.4. Evaluation Cardiotoxicity
Cardiotoxicity is determined according to the following criteria, including chest pain, heartbeat, acute myocardial infarction (AMI), heart failure (HF), and arrhythmia. The patients' follow-up 6 and 12 months after chemotherapy by echocardiography. Then, the data related to echocardiography was evaluated for the detection of cardiotoxicity by the cardiologist.
3.5. Risk Factors for Serious Cardiotoxicity
To evaluate the association between risk factors and cardiotoxicity, we considered several parameters such as a history of smoking, high blood pressure, history of diabetes, history of heart disease, lipid profile, obesity, activity, and Body mass index (BMI). BMI was calculated as the bodyweight in kg by height in m2 (18) TG, CHOL, and HDL were also measured by colorimetric method using the laboratory kits of Pars Azmoon (Tehran, Iran). Also, the Friedewald formula was used for the calculation of LDL (19).
3.6. Statistical Analysis
To describe the data, we used mean (SD) median, and midrange quartiles in quantitative variables; however, frequency and percentage were used in qualitative variables. For single-variable analysis of data, we used t testing (in case of normal data) and Mann-Whitney testing (in case of non-normal data). We performed all analyses using SPSS software, version 22 (SPSS, Inc.). P < 05 was considered to be statistically significant.
4. Results
4.1. Association Between Risk Factors and Ejection Fraction in 6 Months After Treatment
To evaluate the correlation between risk factors and cardiotoxicity, we follow-up function of heart patients in 6 months’ period by echocardiography. The result indicated statistical significant between several risk factors and heart dysfunction. Risk factors include age (P-Value: 0.03), history of heart disease (P-Value: 0.02), history of high blood pressure (P-Value: 0.00), diabetes (P-Value: 0.00), and cholesterol (P-Value: 0.040) (Table 2).
| Risk Factor | EF (N) | P-Value | |
|---|---|---|---|
| ≥ 50 | < 50 | ||
| Age | 0.03 | ||
| 20 - 30 | 0 | 0 | |
| 30 - 40 | 8 | 1 | |
| 40 - 50 | 25 | 2 | |
| > 50 | 20 | 10 | |
| BMI | 0.86 | ||
| ≥ 30 | 15 | 4 | |
| < 30 | 38 | 9 | |
| History of heart disease | 0.02 | ||
| Yes | 4 | 4 | |
| No | 49 | 9 | |
| History of Blood pressure | 0.00 | ||
| ≥ 135/85 | 5 | 11 | |
| < 135/85 | 48 | 2 | |
| History of Diabetes | 0.00 | ||
| FBS ≥ 126 | 7 | 7 | |
| FBS < 126 | 45 | 8 | |
| HDL | 0.12 | ||
| ≥ 40 | 7 | 4 | |
| < 40 | 45 | 9 | |
| LDL | 0.08 | ||
| ≥ 100 | 12 | 6 | |
| < 100 | 41 | 7 | |
| TG | 0.48 | ||
| ≥ 150 | 8 | 45 | |
| < 150 | 3 | 10 | |
| Cholesterol | 0.04 | ||
| ≥ 200 | 10 | 6 | |
| < 200 | 43 | 7 | |
| Physical activity | 0.68 | ||
| Yes | 6 | 2 | |
| No | 47 | 11 | |
| CRP | 0.23 | ||
| ≥ 6 | 3 | 2 | |
| < 6 | 50 | 11 | |
Evaluation of Eject Fraction Six Months After Treatment
4.2. Association Between Risk Factors and Ejection Fraction in 12 Months After Treatment
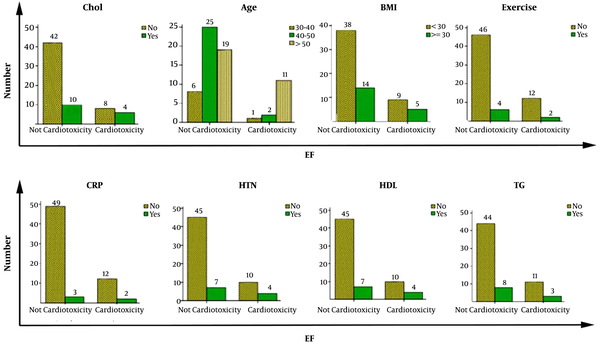
In the second six- month follow-up, heart function was evaluated by echocardiography. The results indicated that the relationship between blood pressure and heart dysfunction was the most significant among the five risk factors investigated in the first six months of follow up (P-Value: 0.01) (Table 3 and Figure 1).
| Risk Factor | EF (N) | P-Value | |
|---|---|---|---|
| ≥ 50 | < 50 | ||
| Age | 0.07 | ||
| 20 - 30 | 0 | 0 | |
| 30 - 40 | 9 | 0 | |
| 40 - 50 | 27 | 0 | |
| > 50 | 25 | 5 | |
| BMI | 0.30 | ||
| ≥ 30 | 17 | 2 | |
| < 30 | 45 | 2 | |
| History of heart disease | 0.01 | ||
| Yes | 7 | 2 | |
| No | 55 | 2 | |
| History of Blood pressure | 0.18 | ||
| ≥ 135/85 | 14 | 2 | |
| < 135/85 | 48 | 2 | |
| History of Diabetes | 0.15 | ||
| ≥ 126 | 13 | 2 | |
| < 126 | 49 | 2 | |
| HDL | 0.65 | ||
| ≥ 40 | 10 | 1 | |
| < 40 | 52 | 3 | |
| LDL | 0.30 | ||
| ≥ 100 | 16 | 2 | |
| < 100 | 46 | 2 | |
| TG | 0.65 | ||
| ≥ 150 | 11 | 1 | |
| < 150 | 51 | 3 | |
| Cholesterol | 0.22 | ||
| ≥ 200 | 14 | 2 | |
| < 200 | 48 | 2 | |
| Physical activity | 0.43 | ||
| Yes | 8 | 0 | |
| No | 54 | 4 | |
| CRP | 0.10 | ||
| ≥ 6 | 4 | 1 | |
| < 6 | 58 | 3 | |
Evaluation of Eject Fraction 12 Months After Treatment
5. Discussion
BC is heterogeneous and has many risk factors involved in its incidence and progression. Studies have shown that many inherited factors such as family history and the occurrence of mutations and acquired factors are essential to play a role in the progression of the disease (20). Today, BC is one of the main reasons of mortality in the world; however, based on the recent therapeutic strategies, the survival of patients were increased (21, 22). Chemotherapy- induced cardiotoxicity is an important problem in cancer treatment (23, 24). The result of this study indicates that cardiotoxicity in cancer patients is associated with several risk factors, including disease history, life style (25). Meredith et al. evaluated the association of the risk factors and anthracyline in pathogenesis cardiomyopathy of acute myeloid leukemia (AML). They indicated that there was no statistical significance among the history of disease (P-Value: 0.4), BMI (P-Value: 0.5), smoking (P-value: 0.9), and blood pressure (P-Value: 0.3) with cardiotoxicity. However, the results indicated that heart dysfunction is less frequent in AML patients who received low dosage anthracycline than the solid tumor patients (26). Kosalka et al. evaluated risk factors such as obesity, dyslipidemia, and diabetes in trastuzumab-induced cardiotoxicity in BC patients. The result indicated that these risk factors could increase the possibility of cardiotoxicity in BC patients (27). Howden et al. showed the effectiveness of exercise on heart function after chemotherapy in BC patients receiving anthracycline. The results indicated that cardiotoxicity is less common in patients who has more exercise before chemotherapy (28). Anber et al. assessed the C-reactive protein (CRP) as a biomarker for detecting cardiotoxicity in BC patients, the result indicated no change in the level of CRP before and after the treatment. Therefore, this factor cannot be used as a biomarker in the detection of cardiotoxicity in BC patients (29). Doyle et al. indicated the presence of cardiovascular disease and heart failure in BC patients receiving chemotherapy could increase the incidence of cardiotoxicity compared with other cases (30). Macgregor et al. showed high blood pressure and cardiovascular disease could increase the incidence of cardiotoxicity in patients who receive chemotherapy (17). In this study, we evaluated the risk factors in inducing cardiotoxicity in BC patients; we followed up heart function in patients after 6 and 12 months of treatment by echocardiography. Risk factors were investigated and listed in Table 1. Six months after chemotherapy, the result showed 5 risk factors, including age (P-Value: 0.03), history of heart disease (P-Value: 0.02), history of blood pressure (P-Value: 0.00), diabetes (P-Value: 0.00) and cholesterol (P-Value: 0.04) statistical significance with induced cardiotoxicity (Table 2). However, 12 months’ follow-up indicated only the history of heart disease was statistically significant. (P-Value: 0.01) (Table 3).
5.1. Conclusion
Finally, chemotherapy can affect many organs, and the most affected organ is the cardiovascular system in BC patients. According to this study, some risk factors that induce cardiotoxicity means make the patient vulnerable to cardiotoxicity during the chemotherapy. So, detecting these factors can be used as a prognostic factor for assessing the situation of the patient before and after treatment.