1. Background
Side effects of androgenic-anabolic steroids (AAS) on the hormonal, metabolic, and nervous systems are the main concern of those who consume such drugs (1). Due to more androgenic properties, such concerns are more frequent among males, and long-term use of AAS causes the accumulation of these drugs or their metabolites in the liver, which in turn increases the likelihood of tumors and hepatitis, cancer, and other liver problems.
AAS is a compound derived from testosterone (the main male hormone) (2) that was widely used in the late 1930s to treat hypogonadism and severe burnout (3). Anabolic steroid hormones are often abused by athletes as an anabolic drug to improve their physical performance. Physiologically, AAS can increase skeletal muscle mass and protein synthesis, and improve muscle size, body mass, and strength. Also, testosterone often referred to as the father of most anabolic-androgenic steroids, leads to the maturation and development of secondary male sexual traits (4). This hormone affects cells by interacting with the central nucleus and causing biochemical changes and, because of its solubility in fat, is dispersed in the cell and, in combination with protein, enters the cell nucleus and activates protein synthesis. The abuse of these drugs is rapidly expanding so that after World War II, athletes have been apparently using anabolic steroid hormones to increase performance (5).
In 1975, the International Olympic Committee recorded steroids on the list of banned drugs and banned its use from that date.
The findings of Jabari et al. (6) regarding the prevalence of anabolic steroid use among athletes in Riyadh showed that most athletes were unaware of its side effects but continued its use.
AAS abuse is associated with a wide range of cardiovascular (7), kidneys, hormonal systems, reproduction, and mental status side effects, with liver damage being the most common (8).
Given that the liver is the largest gland of the human body, complications such as intrahepatic cholestasis, hepatitis, and hepatocellular carcinoma can be noted. These complications are usually associated with alterations in the hepatic function following the use of AAS and an increase in alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and, in some cases, bilirubin (9). Limited studies are conducted on the liver tissue damage and structural changes following the use of anabolic steroids and consequent liver tumors (10). One of the research studies conducted by Rashidlamir et al. (11) is performed on 30 male athletes that were divided into three groups and reported higher degradation of two enzymes (i.e., ALT and AST) in the AAS consumption group compared to the other groups. However, no significant difference was observed between the three groups regarding the ALP enzyme. Tousson Ahab et al. (12) examined the effect of anabolic steroids without physical exercise on rabbit liver and reported inflammation of blood-filled sinusoids and lipid vacuoles in the liver tissue. Also, Boada Lewis et al. (13) examined liver changes in male Wistar rats using oral Winstrol with no exercise and showed that Winstrol can alter the metabolism capacity of hepatocytes in an abnormal way and in large amounts has a greater effect on hepatocytes. The results of studies by Bento-Silva et al. (14) on steroids and endurance training and Flynn et al. (15) showed liver damage in the anabolic steroid consumption groups. Another case study by Chahla et al. (16) on 3 US athletes taking steroids showed liver toxicity and cholestasis (bile retention and accumulation in the liver) and elevated liver enzymes in subjects.
In a study on the side effects of steroids on 20 bodybuilding athletes, Dorry et al. (17) observed that the use of oral steroids oxymetholone and methandrostenolone was associated with increased blood factors, such as hematocrit, hemoglobin, and reticulocyte.
It worth noting that although the adverse effects of these drugs have been clinically investigated in some cases, athletes and other consumers of these drugs still have opposing views with physicians, often taking anabolic steroids and recommending them to others, ignoring the negative consequences of such drugs on physical performance. On the other hand, the mechanism by which the liver is affected by steroids and its potential dangers may be unclear to sports science researchers, so it is difficult to obtain accurate information about the prevalence of AAS due to their illegal use by athletes and the fact that they always try to cover this issue (18). Accordingly, the current study aimed to investigate the effects of anabolic steroids (Winstrol and Oxandrolone) on liver enzymes in male athletes to increase their awareness about the effects of such drugs. The authors hope to take a positive step towards the development of bodybuilding and the scientific promotion of athletes in this field.
2. Objectives
The current study aimed to investigate the effects of anabolic steroids (Winstrol and Oxandrolone) abuse during consumption and after quitting on the liver enzymes in male bodybuilding athletes.
3. Methods
3.1. Participants
This is a causal-comparative study that its statistical population consisted of 30 bodybuilding athletes who were divided into 3 groups (each with 10 members), including (1) athletes with no history of AAS; (2) athletes with a history of AAS (three months away from consumption); and (3) athletes with current AAS consumption (having started taking AAS eight weeks prior to taking blood sample). It is noteworthy that each group had its own volunteers, and the sampling process was performed using the available and non-randomized sampling technique.
Inclusion criteria were regular exercise pattern, having at least 1 year of exercise experience, and no history of traumatic damages, and no history of affective liver enzyme exercise within 48 hours prior to blood sampling. The exclusion criteria were no history of genetic and liver-related diseases and non-use of liver detoxification supplements during steroids withdrawal.
The subjects had regular sleep cycles and no history of smoking or alcohol use.
Par-Q and You questionnaire was used to assess the subjects’ health. Besides, before performing the study, research procedures were explained to the participants in written and oral forms, and written informed consent was obtained from all participants. This article is registered at the Research Committee of Islamic Azad University (code: IR.IAU.KAU.REC.1399.005).
3.2. Procedure
Members of the two experimental groups received Winstrol and Oxandrolone. Few days before the blood sampling, subjects were evaluated for body measurements, and data on the type and amount of AAS use were collected. The subjects were asked to going on a diet defined by the researcher 48 hours prior to sampling and to avoid high-intensity, traumatic exercise. After 12 hours of fasting, 10 mL of blood from the subjects’ cubital vein was taken in a sedentary position. To measure ALP hepatic enzymes, the DGKC method (German Biochemical Society standard), and to evaluate ALT and AST enzymes, the IFCC method (International Federation of Clinical and Laboratory Medicine) was employed, using a Prestin 24i autoanalyzer manufactured by Japan.
3.3. Data Analysis
The data were analyzed using SPSS version 21. After investigating the normality of data by the Kolmogorov-Smirnov test, one-way ANOVA and Tukey post hoc tests were used. A P value of < 0.05 was used as statistical significance.
4. Results
The anthropometric characteristic of the subjects separated by the group is provided in Table 1.
| Groups | Age, y | Height, cm | Weight, kg | BMI, kg/m2 |
|---|---|---|---|---|
| (1) Athletes with no history of AAS | 26.13 | 178.52 ± 8.32 | 79.86 ± 9.94 | 25.19 |
| (2) Athletes with a history of AAS (three months away from consumption) | 26.82 | 177.12 ± 7.85 | 79.86 ± 8.62 | 25.51 |
| (3) Athletes with current AAS consumption | 27.25 | 178.02 ± 9.43 | 82.22 ± 9.75 | 25.94 |
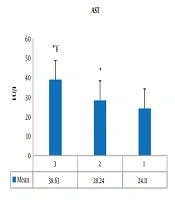
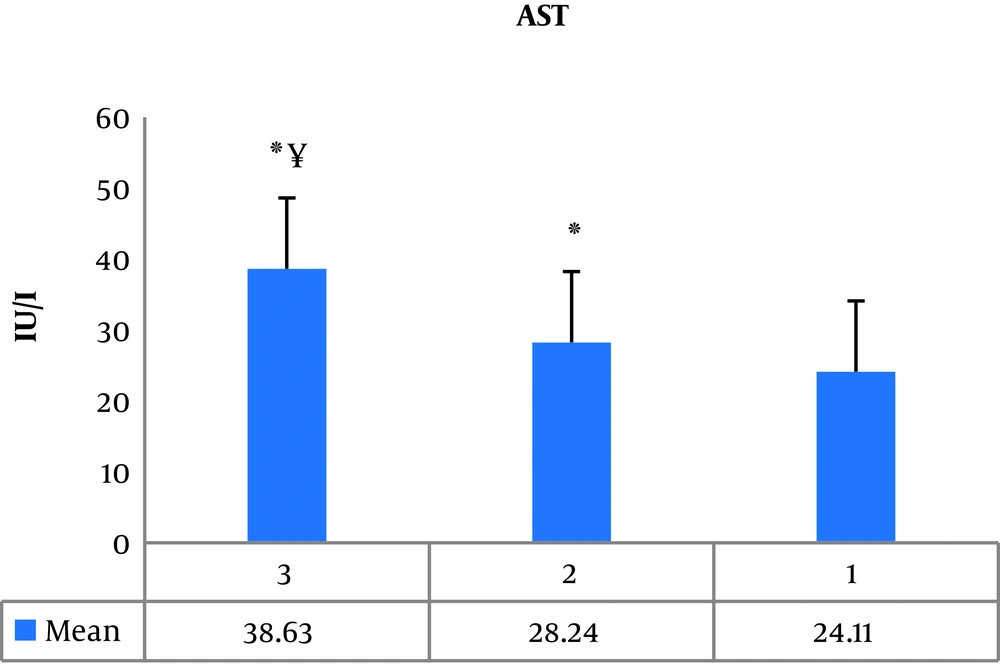
One-way ANOVA showed a significant difference between groups (P = 0.001, F = 130.84). Also, the results of Tukey post hoc test showed a significant increase in serum AST levels in the third group (athletes with current AAS consumption) (P = 0.001) and the second group (athletes with a history of AAS) (P = 0.001) compared to the first group (athletes with no history of AAS). Also, in the third group (P = 0.001) the increase in serum AST levels was significantly higher than the second group (Figure 1).
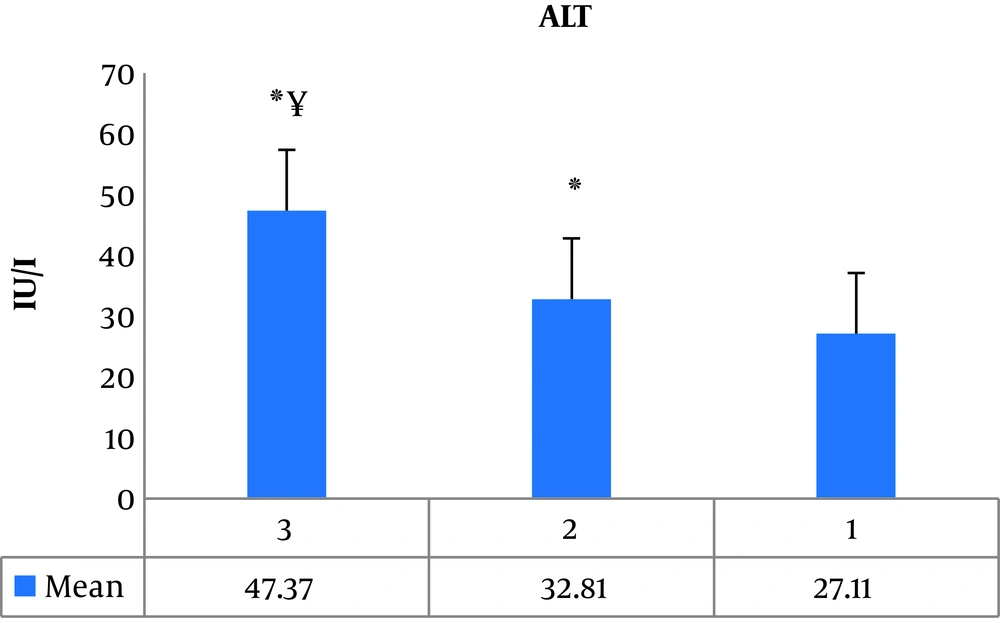
The results of one-way ANOVA showed a significant difference between groups (P = 0.001, F = 188.178). The results of Tukey post hoc test showed a significant increase in ALT levels in the third group (athletes with current AAS consumption) (P = 0.001) and the second group (athletes with a history of AAS) (P = 0.001) compared to the first group (athletes with no history of AAS). Also, the serum ALT level in the third group (P = 0.001) was significantly higher than the second group (Figure 2).
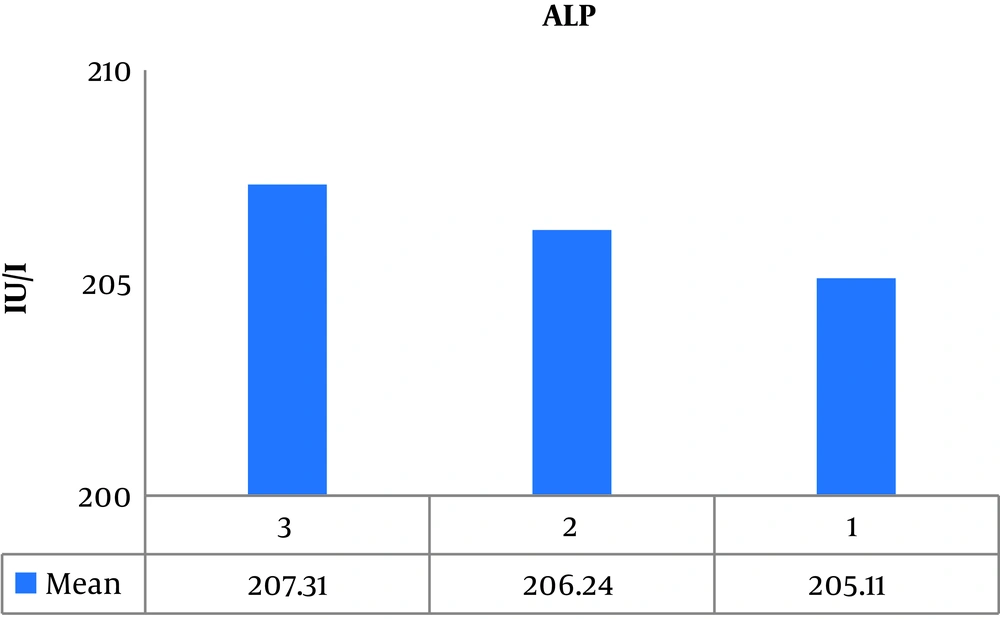
The results of one-way ANOVA showed no significant difference between groups in serum ALP levels. For this reason, no follow-up test was required (P = 0.227, F = 1.349) (Figure 3).
5. Discussion
The current study aimed to evaluate the effects of androgenic anabolic steroids on male bodybuilding athletes. According to the findings, steroids affect the ALT and AST enzymes. On the other hand, the ALP enzyme was not affected by AAS.
AAS is associated with various side effects, and its most important consequence is liver-related consequences (19). According to the literature, the liver is involved in various sports activities and, therefore, its enzymes may be affected by various exercise activities (20). Andrew Parkinson et al. (21), by examining the liver and blood factors of the bodybuilders who used steroid drugs during exercise, found that such drugs dramatically increase the liver enzymes and blood factors, depending on the received dose. Elevated levels of ALT and AST indicate the entry of liver and muscle enzymes into the bloodstream. Therefore, changing the concentration of these enzymes can be due to damage to the liver cells (22). It has also been shown that when damage to the membrane of hepatocyte canaliculi is induced, ALP translocation from the membrane of these canaliculi to the surface of the hepatocytes and, ultimately, to the blood occurs (23).
Since the liver detoxifies many different drugs by chemical changes or excretes them into the bile, steroid hormones that do not reside in the tissues are often converted to androsterone and dihydroepiandrosterone by the liver and immediately conjugated or sulfated and then excreted through the bile into the intestine or through the kidney into the urine. The use of these drugs damages the liver tissue, the severity of which depends on many factors such as type of drug, duration of use and dosage, thus since the liver is the primary site of steroid clearance and detoxification, longer and more serious complications occur in the liver, relative to other organs of the body, which is mainly dependent on the received dose (24).
The findings suggest that the most common tissue damage in the liver is vascular hyperemia, degeneration, inflammation in the liver tissue, and increased cytoplasm fat vacuoles, causing liver deformity and hardening, thereby destroying the liver tissue and replacing it with connective tissue. The created fibrous strands connect areas of the liver and create bridging fibrosis. With fibrosis and parenchyma damage, the normal structure of the liver will be destroyed and cirrhosis will eventually develop. Cirrhosis is an end-stage liver disease that carries the risk of malignancy (25) and, in some cases, may cause liver failure and encephalopathy and eventually, death (26, 27). Therefore, since the liver is the major site of metabolism of steroids, it can be vulnerable to the use of AAS (11). However, it is not precisely clear how AAS causes damage to the liver (28). In this regard, Chahla et al. (16) reported higher levels of ALT and AST in individuals using AAS more than the normal range, and after 12 weeks of discontinuation of these substances, the levels of these enzymes were close to the normal range.
In this regard, the findings reported by Chahla et al. (16) are consistent with the present study. Shahraki and Rafeei (29) conducted a study on 40 male Wistar rats. The results showed no significant difference in the ALT enzyme between the nandrolone steroid receiving group and the control group (29), which is inconsistent with the findings of the present study. A possible reason for the discrepancy may be that the mentioned research was performed on animals, and the experimental subjects received only one type of injectable steroid, while subjects in the present study used two types of oral steroid medications.
There was no statistically significant difference in the levels of ALP enzyme in the present study, which is in line with the findings of Rashidlamir et al. (11), because in this study no significant difference in ALP between the AAS consumption group and the groups with and without AAS is reported. Despite this, in a case study by Socas et al. (30) on two male bodybuilders, the ALP level in subjects was reported to be higher than the normal range, which is not consistent with the findings of the present study. This inconsistency may be due to the number of participants of this study (who were 2 male bodybuilders).
Urhausen et al. (31) examined the reversible effects of AAS on blood cells, lipids, liver function, and hormones in 32 male athletes who were divided into two groups; their results showed that after one-year discontinuation of the drug the negative effects of steroids, including liver dysfunction and some hormones, reverted to the normal state, although in some users (withdrawal group) increased ALT activity and decreased testosterone synthesis were observed.
Therefore, taking AAS intervenes with the normal cycle of testosterone production in the body. Based on Leydig’s findings, by ceasing testosterone production, blood cholesterol is no longer converted to pregnenolone, which raises blood cholesterol. Besides, it is one of the contributing factors to fatty liver (26, 27). Since the fatty liver is associated with inflammation, this leads to the drop of fat droplets into the sinusoids, which is consistent with the findings of Tousson et al. (12). Studies by Friedel et al. (32) and Sinha-Hikim et al. (33) on some abdominal organs such as the intestines, liver, and testicles also showed that taking AAS may have detrimental effects on the intestine, liver, and testicles of adult rats, and can affect their function at higher doses. In testicles, histological findings have shown severe changes such as cellular hypertrophy, degenerative changes, and atrophy, which may be the cause of infertility in these rats (32).
Most studies investigated the biochemistry of athletes’ blood using these drugs have reported that most of the changes were in the AST and ALT. However, no significant changes in ALP levels have been reported (34, 35) as AST and ALP are found more widely in the liver than ALP, so most of the damages caused by steroids are attributed to the two former enzymes (36). Therefore, the most deleterious effects of steroids lie on the liver and liver enzymes, and hence attention to the structure of steroids prepared for edible use is important due to the presence of ethyl or methyl group(s) in their drug structure. The reason is that adding these compounds to the chemical structure of steroids is mainly carried out in the position of carbon 17, which makes the steroids toxic to the liver and can be one of the most important reasons for increased levels of liver enzymes in the blood (37, 38).
Despite the widespread use of these substances among athletes, the chronic detrimental effects of AAS use have been less studied. Also, few information is available on doses of anabolic steroid use in humans. The number of samples used in this study was small because few people were willing to share their sports and steroid use history with researchers, and therefore may not be representative of all long-term AAS users. Also, to determine the type, dose, and duration of steroid use, only the participants’ self-report was sufficient, and the accuracy was not measured. Therefore, it is suggested that researchers in future research should investigate the side effects of androgenic anabolic steroids.
5.1. Conclusions
Overall, according to the results, although the adverse effect of AAS on the liver enzymes decreased significantly over time, but was not eliminated, so that the liver was continuing to be affected even after quitting. Based on the results of the present study and previous studies, it seems that using steroids and the amount of these hormones are important factors in instigating liver damage, which in turn causes hepatic disorder, fatty liver, hepatitis, and hepatic cholestasis. Thus, increasing athletes’ information regarding the consequences of using AAS can be an effective step in preventing the use of these substances and making them less likely to such drugs.