1. Background
Trauma is the first cause of mortality and the main cause of disability in active populations in developing countries (1-3). However, less attention has been paid to this issue in these countries (4). According to the forecasts of the World Health Organization (WHO), by 2024, the accidents will become the second leading cause of lost years of life all around the world (5).
On the other hand, trauma is associated with a high economic and social burden, both direct and indirect. Nowadays, the policies of WHO are based on the preventive measures and care needs of trauma patients (6, 7). Trauma-hemorrhagic shock (THS) is the main cause of traumatic death (8). THS causes activation of myocardial Nuclear factor-kappa B (NF-κB) and tumor necrosis factor alpha (TNFα) (9). Myocardial damage, known as a chronic disease, requires ongoing medical attention. So, THS can induce secondary cardiac injuries (10, 11). These policies not only have resulted in reduced mortality rates attributable to the trauma and socioeconomic burden of the disease but also have improved the outcomes of patients (12, 13).
Quick and appropriate diagnosis and treatment can be effective in improving the outcomes of these patients. The main treatment involves fixation and the patient’s hemodynamic rehabilitation using isotonic fluids and blood products to provide the necessary conditions for surgery or angiography. According to the recent literature, fibrinogen plays a vital role in the acquisition and maintenance of homeostasis, especially in patients with severe blood loss. It seems that plasma fibrinogen concentration, which is a glycoglycoprotein in coagulation falls, is independently associated with hypertension and prophylaxis fibrinogen concentrate infusion (14, 15).
Fibrinogen level has been introduced as a determining factor in the 28-day death prediction of patients with intense and more severe bleeding (16). The injection of fibrinogen concentrate compared with fresh-frozen plasma (FFP) can bring better results concerning improving the outcome and reducing the need for other blood components as well as the enhanced likelihood of survival without increasing the risk of hypobo embolism (17-19).
It seems that factors such as the severity of the trauma, breeding characteristics, underlying diseases, severity of the fracture, and type of fractures contribute to plasma fibrinogen and its reduction in the pursuit of hematological diseases. In any case, the final comment on the effects of fibrinogen injection, instead of products such as FFP, requires more and more accurate studies with regard to other effective variables in the outcome of these patients. However, it seems that fibrinogen has a valuable role in improving the outcomes of patients with severe trauma.
2. Objectives
The current study aimed to investigate the effect of fibrinogen compared to FFP on outcomes of patients with traumatic hemorrhagic shock.
3. Methods
In this double-blind, randomized clinical trial study, hemorrhagic shock patients (caused by multiple traumas) with chronic diseases referred to the emergency department (ED) of Golestan Hospital, Ahvaz (Iran) are investigated. First, patients were examined by a physician for both clinical assessment and physical examination.
All steps of the study were performed following the Helsinki ethical principles after receiving the code of ethics, clinical trial code (code: IRCT20150704023046N4, https://www.irct.ir), and informed consent to participate in the study.
3.1. Inclusion Criteria
Patients with hemorrhagic shock (caused by multiple trauma), older than 18 years, systolic blood pressure < 90 mmHg, the possibility of significant bleeding, intra-abdominal bleeding evidence, and patients who need blood products according to the clinical conditions.
3.2. Exclusion Criteria
Being pregnant, having known coagulopathy, history of receiving anticocagan and antiplatelet drugs, other causes of shock such as neurogenic shock, having a history of receiving blood products during the last 6 months, and dissatisfaction to participate in the study.
3.3. Intervention
The first group was treated with fibrinogen extract, the second group received FFP, and the third group underwent a routine treatment (i.e. crystalloid and packed red blood cells (PRBC)). Seventy mg/kg of fibrinogen (Avin Darou Company, Tehran, Iran) was injected, and FFP in proportion (FFP: PRBC ratio 1:1) was performed.
3.4. Outcome
3.4.1. Primary
The vital signs of patients were recorded in their records, and the patient’s blood pressure was recorded at a distance of 3 to 5 minutes. In assessing the effectiveness of each of these therapies in controlling coagulation disorders and outcomes of patients, blood pressure, pH with arterial blood gas (ABG) test, base excess level, and oxygenation index with pulse oximetry were recorded 4 and 12 hours after admission.
3.4.2. Secondary
Information such as brain injuries and organs in the short- (24 hours), medium- (7 days), and long-term (28 days), or discharge from hospital (with the level of consciousness and discharge time) were recorded. Also, the need for admission in special departments, the number of infused blood units, the occurrence of multiple cases of failure, and duration of hospitalization, and death were recorded.
3.5. Randomization
Boxes that contained the name of medications were labeled and classified based on a table of random numbers using computer software. Only the pharmacist and statistician of the research team were aware of the boxes. Finally, all coded medicine boxes were turned over. Based on the method of blocks, four subjects were randomly divided into three equal groups.
3.6. Blinding
In this study, the physician and patients were not aware of the intervention, and injections were performed by a third person.
3.7. Sample Size
In this study, the sample size was calculated using a literature review (20), and the minimum size of the statistical populations was calculated as 32.
3.8. Statistical Analysis
After collecting the data, SPSS (V 20) was used to analyze the data. The findings are described using the mean (standard deviation), frequency, and percentage. To analyze the quantitative variables, ANOVA or Kruskal-Wallis tests were used. The chi-Square test was used to compare the data. Statistical significance was considered when P-value < 0.05.
4. Results
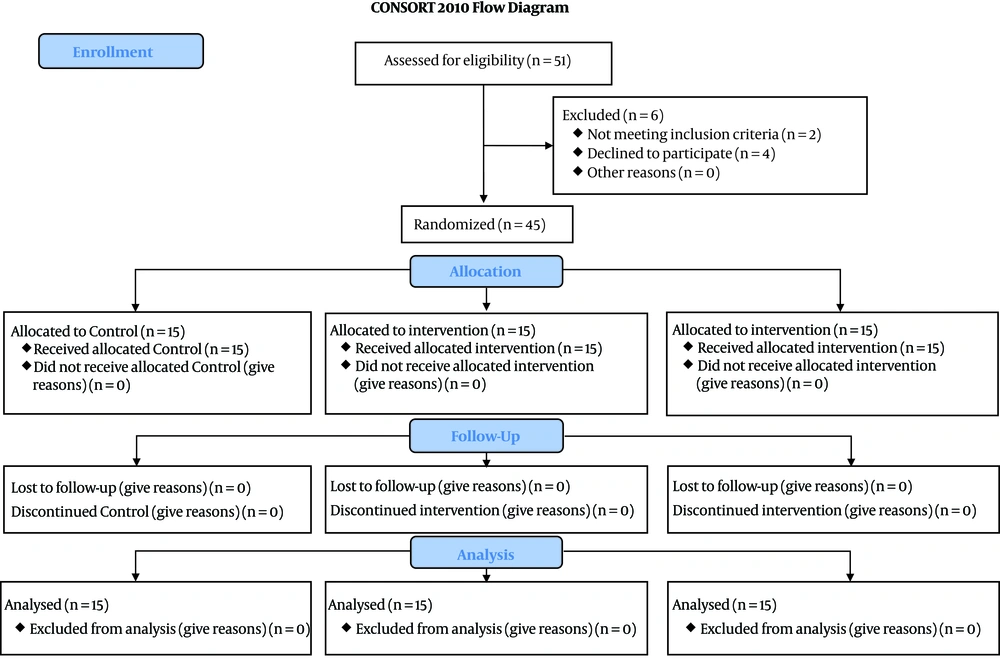
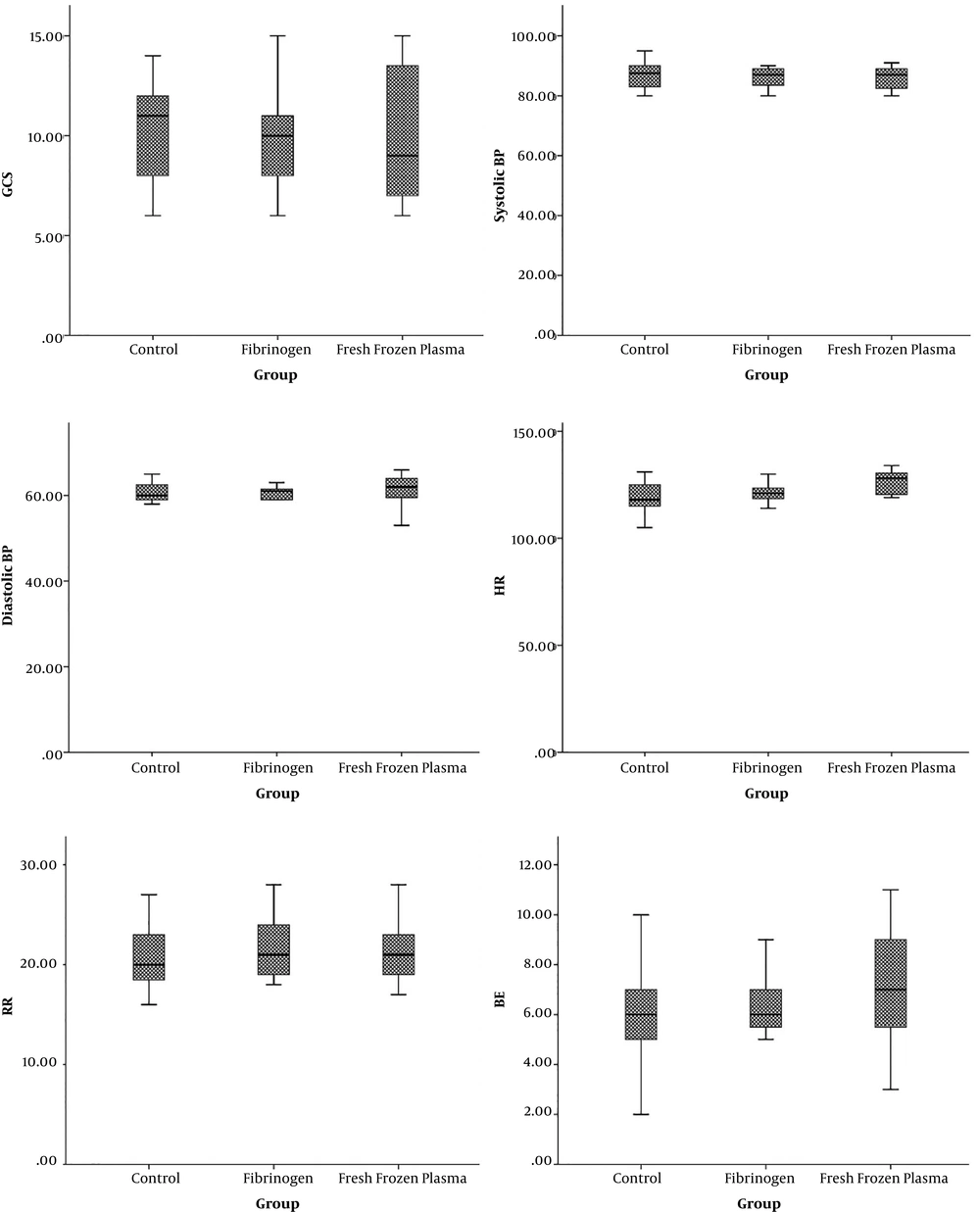
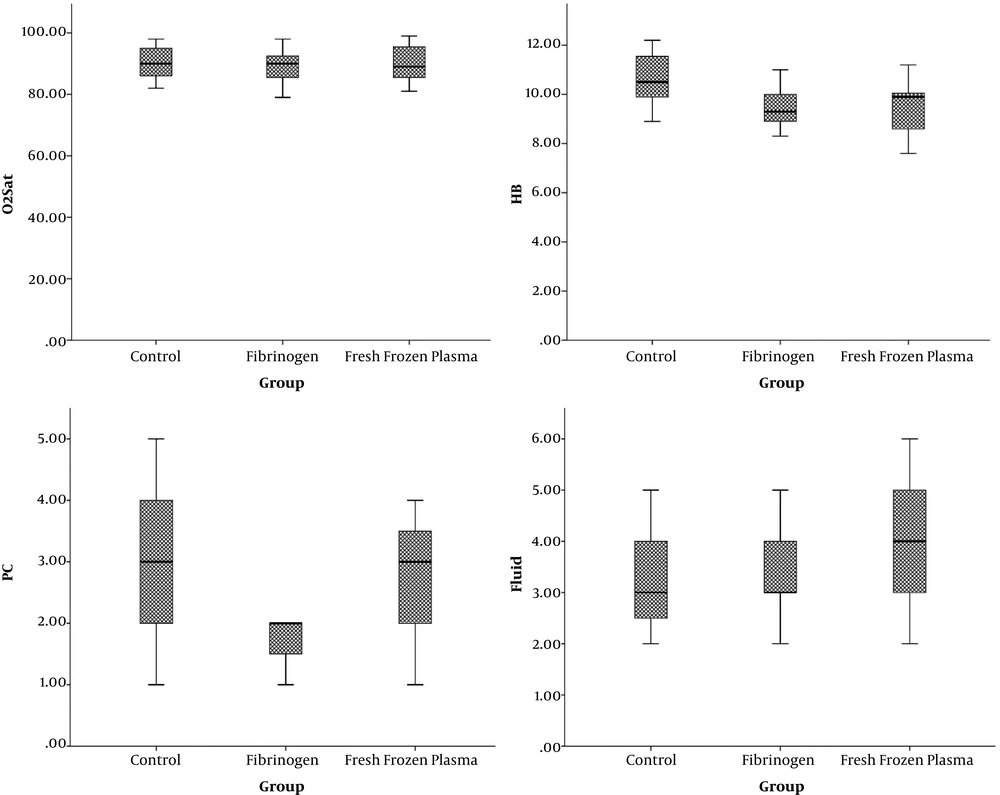
In this study, 45 patients were studied (Figure 1). The mean age of patients was 42 ± 13.8, ranging from 20 to 60 years. Most of the participants were male (33 or 73.3%) (Table 1). The mean Glasgow Coma scale (GCS) of the three groups was similar. There was no significant difference in systolic and diastolic blood pressure, heart rate, respiratory rate, pH level, and O2Sats of patients in all three groups. However, hemoglobin level was significantly higher in patients who received crystallization (Table 2) (Figures 2 and 3).
| Characteristics | Control (N = 15) | Fibrinogen (N = 15) | Fresh Frozen Plasma (N = 15) | P-Value |
|---|---|---|---|---|
| Age, Mean ± SD | 41.13 ± 14.34 | 42.33 ± 13.88 | 42.73 ± 14.30 | 0.95 |
| Gender, No. (%) | 0.25 | |||
| Male | 13 (86.7) | 11 (73.3) | 9 (60) | |
| Female | 2 (13.3) | 4(26.7) | 6 (40) | |
| Traumas site, No. (%) | ||||
| Head | 8 (42.1) | 11 (37.9) | 10 (34.5) | 0.7 |
| Abdomen | 14 (50) | 8 (28.6) | 6 (21.4) | 0.9 |
| Limb | 9 (40.9) | 10 (45.45) | 3 (13.63) | 0.32 |
| Chest | 5 (25) | 13 (65) | 2 (10) | 0.04 |
| GCS, Mean ± SD | 10.20 ± 2.48 | 10 ± 2.53 | 10 ± 3.31 | 0.97 |
| Characteristics | Control (N = 15) | Fibrinogen (N = 15) | Fresh Frozen Plasma (N = 15) | P-Value |
|---|---|---|---|---|
| Systolic BP | 85.86 ± 5.75 | 86.06 ± 3.39 | 86.2 ± 4.0 | 0.97 |
| Diastolic BP | 60.26 ± 3.57 | 59.46 ± 3.44 | 61.2 ± 4.27 | 0.46 |
| HR | 119.26 ± 7.85 | 121.6+4.47 | 126.33 ± 5.63 | 0.01 |
| RR | 19.95 ± 4.57 | 21.73 ± 3.20 | 21.47 ± 3.25 | 0.37 |
| PH | 7.3 ± 0.034 | 7.28 ± 0.05 | 7.29 ± 0.05 | 0.66 |
| BE | 6.0 ± 2.13 | 6.06 ± 2.19 | 7.13 ± 2.33 | 0.3 |
| O2Sat | 90.40 ± 5.7 | 89.46 ± 5.19 | 90.27 ± 5.90 | 0.8 |
| HB | 10.6 ± 1.08 | 9.48 ± 0.73 | 9.38 ± 1.02 | 0.002 |
| PC | 2.93 ± 1.09 | 2 ± 0.8 | 2.7 ± 1.03 | 0.035 |
| Fluid | 3.2 ± 0.9 | 3.26 ± 0.79 | 4.1 ± 1.4 | 0.04 |
| Hospitalization | 16.3 ± 7.18 | 13 ± 4.6 | 15.2 ± 5.89 | 0.31 |
| ICU | 13 (86.7) | 11 (73.3) | 14 (93.3) | 0.3 |
| Ventilation | 8 (46.7) | 7 (46.7) | 9 (60) | 0.7 |
| Multiple organs | 2 (13.3) | 2 (13.3) | 4 (26.7) | 0.54 |
aValues are expressed as No. (%) or mean ± SD.
The number of RBCs in patients treated with crystalloid, FFP, and fibrinogen was 2.93, 2.37, and 2, respectively, which the difference was statistically significant (P = 0.03). The mean days of hospitalization in FFP, fibrinogen, and crystalloid groups was 15, 13, and 16.3 days, respectively (P = 0.3). The proportion of individuals requiring hospitalization in each of the three groups did not show any difference (P = 0.3). Also, the need for ventilation was similar in all three groups. Two patients (13.3%) in the fibrinogen group, 4 (26%) in the FFP group, and 2 in the control group (P = 0.5). The incidence rate of sepsis was significantly higher in the FFP group. In contrast, the mortality rate in the crystallization group (46.7%) was significantly higher than the rest of the groups. The lowest mortality rate was observed in the fibrinogen group (P = 0.13) (Table 3).
| Characteristics, No. (%) | Control (N = 15) | Fibrinogen (N = 15) | Fresh Frozen Plasma (N = 15) | P-Value |
|---|---|---|---|---|
| ICU | 13 (86.7) | 11 (73.3) | 14 (93.3) | 0.3 |
| Ventilation | 8 (46.7) | 7 (46.7) | 9 (60) | 0.7 |
| Multiple organ | 2 (13.3) | 2 (13.3) | 4 (26.7) | 0.54 |
| Adverse effect (AE) | ||||
| Sepsis | 3 (20) | 3 (20) | 8 (53.3) | 0.07 |
| Mortality | 7 (46.7) | 2 (13.3) | 5 (33.3) | 0.139 |
5. Discussion
According to the findings, the number of RBC injection in patients treated with fibrinogen was significantly lower than patients who received FFP and those in the control group. Given that the primary Hb level in patients treated with fibrinogen was lower, probably some products were injected to improvement for the initial level of Hb, and no modification of the product had been injected.
The findings also showed that the incidence of mortality and multiple limb defects in FFP and the control group were more than the fibrinogen group. However, the differences were not statistically significant. These findings were also related to other studies in this regard.
Nienaber et al. (21), in a comparative study of the comparison of FFP and the concentration of coagulation factor on the mortality and morbidity of trauma and bulking bleeding, reported a difference in the general mortality level between the two groups. However, there was a significant difference concerning the morbidity and the need for allogeneic injection. In fact, the findings of this study showed that management of traumatic patients was better with coagulation factors than by FFP injection (21).
Wafaisade et al. (22) evaluated the effect of the administration of fibrinogen concentrate for improving patients with severe trauma and showed that the early use of fibrinogen concentrate was associated with significant improvement in reducing six-hour mortality and also increasing the time until the occurrence of death. However, patients who received fibrinogen showed a higher rate of multiple limb defects (22).
Akbari et al. also evaluated the effect of fibrinogen in comparison with FFP for the outcome of traumatic patients and showed that the use of fibrinogen caused a significant reduction in mortality, sepsis, and the need for RBC product injection (23). Minimizing or refusing to deal with the blood products is highly favorable. FFP injection always increases the risk for health problems such as pulmonary injury following blood transfusion, blood circulation, acute respiratory distress syndrome, immune modulation, and pathogen transmission (24, 25). A few amounts of FFP is not effective in correcting the condition of the patient’s coagulation. Therefore reducing the injection of FFP units is not reversible (26, 27).
Therefore, FFP injection should be prescribed at a high rate, which also increases the influence over hematocrit, and therefore the need for RBC injection. On the other hand, through increasing plasma fibrinogen levels, fibrinogen infusion increases the level of clots, even in people with low platelet levels. Besides, it may reduce the platelets (28, 29).
Moreover, the concentrate of coagulation factors may cause faster blood cessation and decreased hemodialysis compared to the blood allogeneic products. In addition to the reduction of injection volume, a decrease in coagulation factors will probably have less effect on the amount of hematopoietic, and it can be improved in the formation of clots (30).
Moreover, the concentration of coagulation factors in the production process, which is placed under the virus infection and the risk of transmission of viral infections in these products is nearly zero. On the other hand, the risk of transmission of blood-borne infections in plasma products should always be considered as a serious risk. Therefore, fibrinogen concentrate, in addition to improving the outcomes of patients, decreases the risk of infection transmission, and therefore it can be suggested as a beneficial substitute for FFP in multiple trauma.
5.1. Limitations
The current research was a pilot study, and few samples were investigated. The study was carried out in only one center. Symptoms of blood transfusion were not considered. Hence, Randomized Clinical trials are recommended to confirm the findings of the present study.
5.2. Conclusions
This study demonstrated that treating patients with fibrinogen concentrate was associated with reduced mortality and the need for RBC products. Therefore, it seems that management of traumatic patients with fibrinogen, while reducing patient exposure to blood allogenic products, can improve treatment outcomes of patients with multiple trauma.