1. Background
A novel coronavirus disease 2019 (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) that has a 79.5% similarity to SARS-CoV (SARS epidemic in 2003) and spread in individuals through various routes, such as droplets, airborne particles, feces, and oral mucosa (1, 2). Patients with COVID-19 have shown a wide range of symptoms, including asymptomatic to respiratory, gastrointestinal, neurological, etc. The most common clinical symptoms were fever, cough, and fatigue. Gastrointestinal symptoms such as nausea, anorexia, diarrhea, and vomiting are common, and even some patients have experienced gastrointestinal symptoms without respiratory symptoms (3, 4). The COVID-19 mortality rate at initial studies in China has been reported to be 2.3% (4). Further, On December 04, 2020, has proceeded in more than 165,000 dying worldwide with a universal mortality rate of 6.8%, and at this time, December 04, 2020, globally mortality reported by WHO is 2.3% (5). In July 2020, an experiment in Wuhan, China, revealed that older age, hypertension, and elevated lactate dehydrogenase (LDH) need accurate detection and immediate interference to stop the possible development of rigorous COVID-19. Severe male cases with heart damage, hyperglycemia, and high-dose corticosteroid use may be in great danger of death (6). An experiment in Italy revealed that of 3,988 critically ill patients admitted from February 20 to April 22, 2020, 50.4% of patients with COVID-19 had been discharged from the intensive care unit, 48.7% had died in the intensive care unit, and 0.8% were still in intensive care units (ICU) (7).
2. Objectives
Given the fact that the symptoms of the disease, its pathogenicity, and other features can be different in various situations and places, we aimed to compare the epidemiological, clinical, and paraclinical features of patients with COVID-19 who have overcome the disease with those patients who died in Ahvaz, Southwest of Iran.
3. Methods
All adult patients admitted to Special Corona Center with a diagnosis of COVID-19 over three months were included in this study. The current observational, retrospective investigation evaluated all hospitalized patients from January to March 2020 in Ahvaz city. Having clinical symptoms of COVID-19 and also positive real-time PCR for SARS-CoV-2 were the inclusion criteria. Therefore, subjects with negative laboratory results of SARS-CoV-2 were excluded from the study. All patients in this study lived in Ahvaz city during the COVID-19 outbreak. Demographic data, clinical characteristics (including medical history, history of exposure, symptoms, and laboratory findings) were extracted from each patient's medical records. The date of disease onset and hospital admission date, and the severity of COVID-19 were also noted. The onset date was defined as the day when the patients noticed any symptoms. The severity of COVID-19 was defined according to the diagnostic and treatment guideline for SARS-CoV-2 issued by the Chinese National Health Committee version 3 - 5 (8). The Ethical Approval Code is IR.AJUMS.REC.1399.088.
3.1. Statistical Analysis
Independent sample t-test was used to compare continuous variables between the groups of the discharged and expired patients. The independence between categorical variables and the outcome was assessed by chi-square or Fisher's exact tests. The Kaplan-Meyer curve was plotted to visualize the development of survival probabilities for two different starting time-points, hospital admission and clinical symptoms diagnosis. Moreover, the log-rank test was used to investigate the difference in the two starting time-point curves' survival probabilities. Survival analysis was utilized to assess the impact of various variables on time to death/discharge data. In this dataset, dying from COVID-19 was considered the event, and discharge was assumed to be the right censoring. When the number of covariates and factors exceeds the number of observations, routine and standard survival analysis approaches, such as Cox's proportional hazard regression, do not result in adequate and reliable estimations (9).
4. Results
Of all 97 cases with COVID-19, 30 (30.9%) died, and 67 (69.1%) were discharged after recovery. The distribution of variables across the two groups of cases is shown in Table 1 and 2. Death from COVID-19 was significantly associated with critical disease intensity (P < 0.001), loss of consciousness (P = 0.001), ischemic heart disease (IHD) (P = 0.005), Parkinson (p = 0.028), invasive O2 support (P < 0.001), and non-negative Troponin (P = 0.016). Dead individuals were almost 11 years older than those discharged (P = 0.001). Discharging from COVID-19 was associated with the lower mean of respiratory rate (RR), blood sugar (BS), BUN, AST, total and direct bilirubin, neutrophil count, and sodium. Moreover, discharging is affiliated with higher O2 saturation, higher lymphocyte count, and neutral pH, higher HCO3, and base excess (BE) ( Table 2).
| Variable | Outcome, No. (%) | P-Value | |
|---|---|---|---|
| Discharge, 67 (69.1%) | Death, 30 (30.9%) | ||
| Gender | 0.812 | ||
| Female | 24 (35.80) | 10 (33.30) | |
| Male | 43 (64.20) | 20 (66.70) | |
| Tobacco and alcohol | 5 (7.50) | 4 (13.30) | 0.452 |
| Sign and Symptoms | |||
| Cough | 55 (82.10) | 20 (66.7) | 0.118 |
| Dyspnea | 26 (38.80) | 15 (50.00) | 0.302 |
| Orthopnea | 2 (3.0) | 0 (0.0) | 0.999 |
| Paroxysmal nocturnal dyspnea (PND) | 2 (3.0) | 0 (0.0) | 0.999 |
| Sore throat | 4 (6.00) | 0 (0.00) | 0.308 |
| Chest pain | 4 (6.00) | 1 (3.30) | 0.677 |
| Fever | 46 (68.70) | 13 (43.30) | 0.018 |
| Chills | 29 (43.30) | 7 (23.30) | 0.060 |
| Tachypnea | 2 (3.00) | 0 (0.00) | 0.999 |
| Loss of speech | 0 (0.00) | 1 (3.30) | 0.309 |
| Dizzying | 3 (4.50) | 0 (0.00) | 0.550 |
| Runny nose | 1 (1.50) | 0 (0.00) | 0.999 |
| Level of consciousness (LOC) | 0 (0.00) | 6 (20.00) | 0.001 |
| Hyperhidrosis | 3 (4.50) | 1 (3.30) | 0.793 |
| Weakness | 19 (28.40) | 8 (26.70) | 0.864 |
| Lethargy | 19 (28.40) | 8 (26.70) | 0.864 |
| Sleepiness | 0 (0.00) | 1 (3.30) | 0.309 |
| Hemoptysis | 1 (1.50) | 0 (0.00) | 0.999 |
| Myalgia | 27 (40.30) | 7 (23.30) | 0.106 |
| Vomiting | 6 (9.00) | 3 (10.00) | 0.870 |
| Nausea | 14 (20.90) | 4 (13.30) | 0.376 |
| Anorexia | 9 (13.40) | 5 (16.70) | 0.675 |
| Constipation | 2 (3.00) | 1 (3.30) | 0.927 |
| Diarrhea | 8 (11.90) | 2 (6.70) | 0.430 |
| Stomachache | 1 (1.50) | 1 (3.30) | 0.550 |
| Dry mouth | 1 (1.50) | 0 (0.0) | 0.999 |
| Delusion | 0 (0.0) | 1 (3.30) | 0.309 |
| Confusion | 0 (0.00) | 1 (3.30) | 0.309 |
| Headache | 14 (20.90) | 2 (6.70) | 0.137 |
| Past medical history | |||
| Coronary artery bypass graft (CABG) | 5 (7.50) | 4 (13.30) | 0.357 |
| Other operations | 3 (4.50) | 3 (10.0) | 0.297 |
| Operation mediastinum | 1 (1.50) | 0 (0.0) | 0.999 |
| Chronic obstructive pulmonary disease (COPD) | 2 (3.00) | 1 (3.30) | 0.927 |
| Diabetes mellitus (DM) | 15 (22.40) | 11 (36.70) | 0.142 |
| Hypertension (HTN or HT) | 21 (31.30) | 11 (36.70) | 0.606 |
| Heart failure | 2 (3.00) | 3 (10.00) | 0.149 |
| Ischemic heart disease (IHD) | 6 (9.00) | 9 (30.00) | 0.005 |
| Dengue hemorrhagic fever (DHF) | 1 (1.50) | 1 (3.30) | 0.525 |
| Cerebrovascular accident (CVA) | 1 (1.50) | 3 (10.00) | 0.086 |
| Congestive heart failure (CHF) | 0 (0.00) | 1 (3.30) | 0.309 |
| Hyperlipidemia | 3 (4.50) | 2 (6.70) | 0.643 |
| Sinusitis | 1 (1.50) | 0 (0.00) | 0.999 |
| End-stage renal disease (ESRD) | 2 (3.0) | 0 (0.0) | 0.999 |
| Chronic kidney disease (CKD) | 1 (1.50) | 2 (6.70) | 0.225 |
| Asthma | 5 (7.50) | 0 (0.00) | 0.320 |
| Pneumonia | 0 (0.00) | 1 (3.30) | 0.309 |
| Allergy | 1 (1.50) | 0 (0.00) | 0.999 |
| Tuberculosis (TB) | 0 (0.00) | 1 (3.30) | 0.309 |
| Fatty liver | 1 (1.50) | 1 (3.30) | 0.525 |
| Bedridden | 0 (0.00) | 1 (3.30) | 0.309 |
| Cardiomegaly | 0 (0.00) | 1 (3.30) | 0.309 |
| Hyperthyroidism | 2 (3.00) | 0 (0.00) | 0.999 |
| Rheumatoid arthritis | 1 (1.50) | 0 (0.00) | 0.999 |
| Acute kidney injury (AKI) | 0 (0.00) | 2 (6.70) | 0.093 |
| Auto Immune hepatitis | 1 (1.50) | 0 (0.00) | 0.999 |
| Parkinson | 0 (0.00) | 3 (10.00) | 0.028 |
| Gout | 1 (1.50) | 0 (0.00) | 0.999 |
| Human immunodeficiency viruses (HIV) | 0 (0.00) | 1 (3.30) | 0.309 |
| Pacemaker | 1 (1.50) | 0 (0.00) | 0.999 |
| Kidney transplant patients | 1 (1.50) | 0 (0.00) | 0.999 |
| Critical criterion | |||
| Ventilator | 1 (1.50) | 15 (50.0) | <0.001 |
| Shock | 0 (0.00) | 1 (3.30) | 0.999 |
| ICU/ multi organ failure | 2 (3.00) | 5 (16.70) | 0.606 |
| Ventilator & multi organ failure | 64 (95.5) | 9 (30.0) | 0.018 |
| O2 support | <0.001 | ||
| Invasive | 3 (4.50) | 25 (83.30) | |
| Noninvasive | 17 (25.40) | 5 (16.70) | |
| Spontaneous | 47 (70.10) | 0 (0.00) | |
| Treatments | |||
| Antiviral | 59 (88.1) | 20 (66.7) | 0.012 |
| Antibiotic | 42 (62.70) | 25 (83.30) | 0.042 |
| Corticosteroid | 54 (80.60) | 22 (73.30) | 0.422 |
| Positive troponin | 0 (0.00) | 3 (15.80) | 0.016 |
| Aware of the transmission source | 9 (13.40) | 4 (13.30) | 0.989 |
| Disease intensity | |||
| Weakly | 0 (0.00) | 1 (5.30) | 0.999 |
| Mild | 50 (74.6) | 0 (0.00) | 0.001 |
| Severe | 14 (20.9) | 1 (3.3) | 0.001 |
| Critical | 3 (4.5) | 29 (96.7) | 0.001 |
| Outcome | Mean (SD) | P-Value |
|---|---|---|
| Creatine kinase-MB (CK-MB) | 0.792 | |
| Discharge | 22.750 (19.441) | |
| Death | 25.000 (10.412) | |
| Respiratory rate | 0.043 | |
| Discharge | 23.552 (7.163) | |
| Death | 26.767 (7.016) | |
| Age | 0.001 | |
| Discharge | 51.930 (15.088) | |
| Death | 62.830 (15.295) | |
| O2 Sat. | < 0.001 | |
| Discharge | 94.896 (4.537) | |
| Death | 87.000 (11.117) | |
| Blood sugar | 0.020 | |
| Discharge | 130.091 (82.084) | |
| Death | 204.286 (124.959) | |
| Creatinine | 0.329 | |
| Discharge | 1.603 (2.442) | |
| Death | 2.097 (1.882) | |
| BUN | < 0.001 | |
| Discharge | 19.761 (14.075) | |
| Death | 46.933 (41.666) | |
| Aspartate aminotransferase (AST) | 0.052 | |
| Discharge | 50.344 (34.088) | |
| Death | 155.643 (417.416) | |
| Alanine aminotransferase (ALT) | 0.154 | |
| Discharge | 32.361 (35.944) | |
| Death | 50.750 (85.117) | |
| Total bilirubin | 0.016 | |
| Discharge | 0.995 (0.471) | |
| Death | 1.311 (0.725) | |
| Direct bilirubin | 0.035 | |
| Discharge | 0.300 (0.350) | |
| Death | 0.536 (0.685) | |
| Alkaline phosphatase | 0.472 | |
| Discharge | 195.684 (121.418) | |
| Death | 173.333 (69.822) | |
| Lactate dehydrogenase (LDH) | 0.216 | |
| Discharge | 596.072 (274.847) | |
| Death | 686.134 (323.019) | |
| WBC count | 0.165 | |
| Discharge | 8.065 (9.220) | |
| Death | 10.663 (6.313) | |
| Neutrophil count | < 0.001 | |
| Discharge | 67.003 (12.973) | |
| Death | 77.323 (10.445) | |
| Lymphocyte count | < 0.001 | |
| Discharge | 27.024 (13.045) | |
| Death | 15.847 (8.364) | |
| RBC count | 0.127 | |
| Discharge | 4.509 (0.566) | |
| Death | 4.295 (0.741) | |
| Hemoglobin | 0.071 | |
| Discharge | 13.021 (1.717) | |
| Death | 12.260 (2.238) | |
| Hematocrit | 0.139 | |
| Discharge | 37.975 (4.626) | |
| Death | 36.197 (6.631) | |
| Platelet count | 0.458 | |
| Discharge | 171.726 (54.449) | |
| Death | 161.778 (65.304) | |
| Prothrombin Time (PT) | 0.144 | |
| Discharge | 12.586 (2.527) | |
| Death | 13.423 (2.038) | |
| Partial Thromboplastin Time (PTT) | 0.452 | |
| Discharge | 37.426 (18.649) | |
| Death | 40.423 (11.197) | |
| International normalized ratio (INR) | 0.077 | |
| Discharge | 1.141 (0.288) | |
| Death | 1.272 (0.334) | |
| Erythrocyte sedimentation rate (ESR) | 0.281 | |
| Discharge | 42.490 (25.876) | |
| Death | 51.444 (39.057) | |
| pH | < 0.001 | |
| Discharge | 7.404 (0.057) | |
| Death | 7.288 (0.194) | |
| PCO2 | 0.942 | |
| Discharge | 44.321 (8.284) | |
| Death | 44.145 (13.913) | |
| HCO3 | 0.001 | |
| Discharge | 26.135 (4.202) | |
| Death | 21.919 (6.369) | |
| Na | 0.010 | |
| Discharge | 135.739 (2.769) | |
| Death | 138.267 (6.565) | |
| K | 0.828 | |
| Discharge | 4.099 (0.564) | |
| Death | 4.130 (0.823) | |
| P | 0.441 | |
| Discharge | 4.267 (1.791) | |
| Death | 5.290 (2.813) | |
| Ca | 0.739 | |
| Discharge | 9.057 (1.162) | |
| Death | 8.900 (0.811) | |
| Mg | 0.328 | |
| Discharge | 2.025 (0.287) | |
| Death | 2.550 (0.943) |
The survival probability quartiles in two different starting times of admission and presentation of symptoms are shown in Table 3. The starting time for admission was recorded for all patients, while only 77 (79%) cases remembered the day when the first COVID-19 symptoms appeared. Based on the Kaplan-Meier (Product Limit) approach, the mean survival time with admission and beginning of symptom as the starting times was 11.92 days and 20.87 days, respectively. Moreover, 25% of the cases survived 26 days and 17 days after the beginning of symptoms and admission, respectively. The median and third quartile survival time after admission was 12 days and eight days, respectively. The median and third quartile survival time after symptoms were 22 days 16 days, respectively. In other words, 50% of the cases died between days 16 and 26 after diagnosing their clinical symptoms. Also, half of the patients died between days eight and 17 after their first admission. The log-rank test showed a significant difference in the two survival probabilities (chi-square = 17.39, DF = 1, P < 0.001).
| Quantity and Start From | Estimate Hour (Day) | Std. Error | 95% Confidence Interval | |
|---|---|---|---|---|
| Mean | ||||
| Admission | 286.119 (11.92) | 25.933 | 235.29 | 336.949 |
| Symptom | 500.899 (20.87) | 40.712 | 421.103 | 580.695 |
| First quartile | ||||
| Admission | 408 (17) | 55.118 | ||
| Symptom | 624 (26) | 69.561 | ||
| Median | ||||
| Admission | 288 (12) | 24.79 | ||
| Symptom | 528 (22) | 35.195 | ||
| Third quartile | ||||
| Admission | 192 (8) | 29.869 | ||
| Symptom | 384 (16) | 39.123 | ||
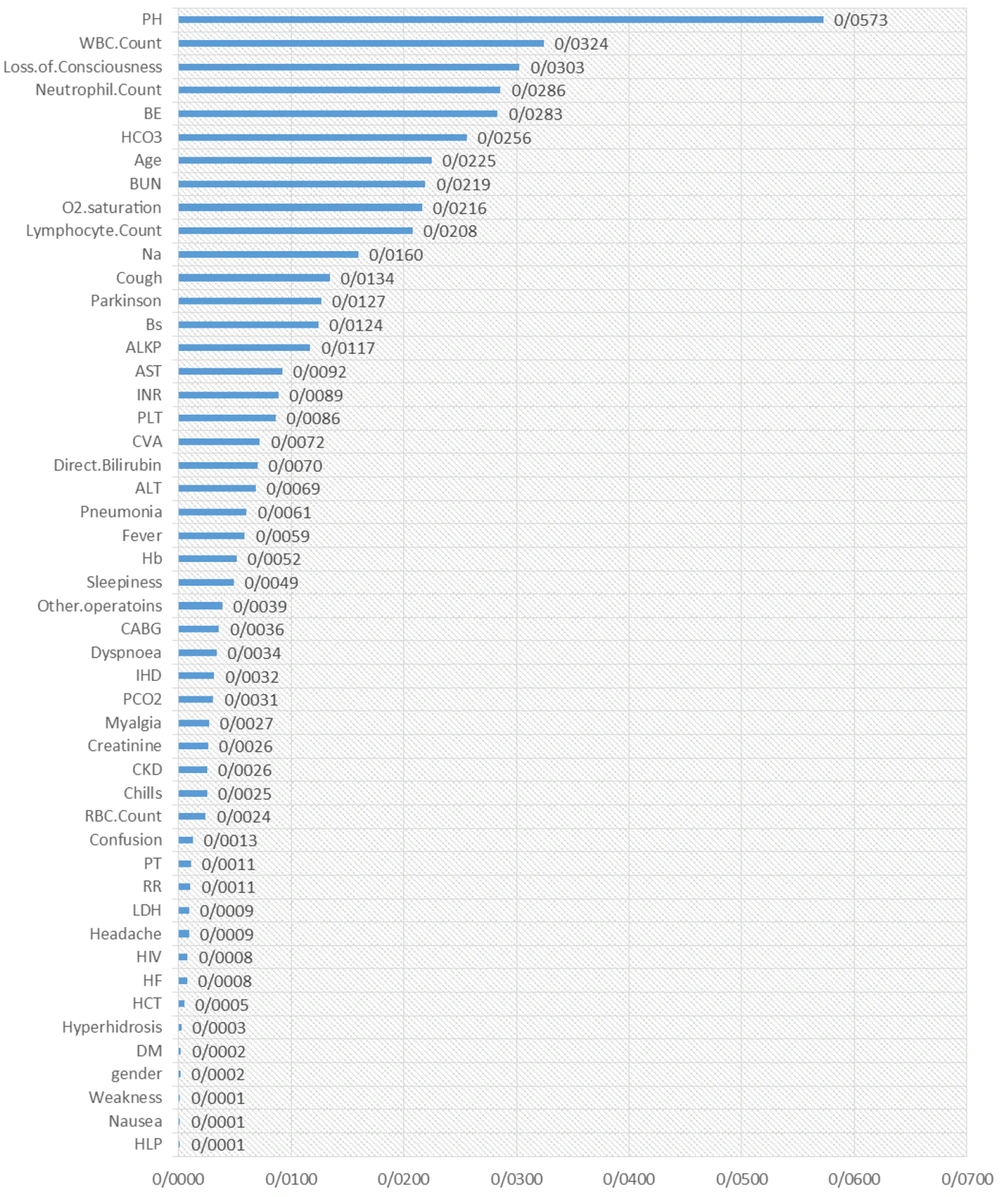
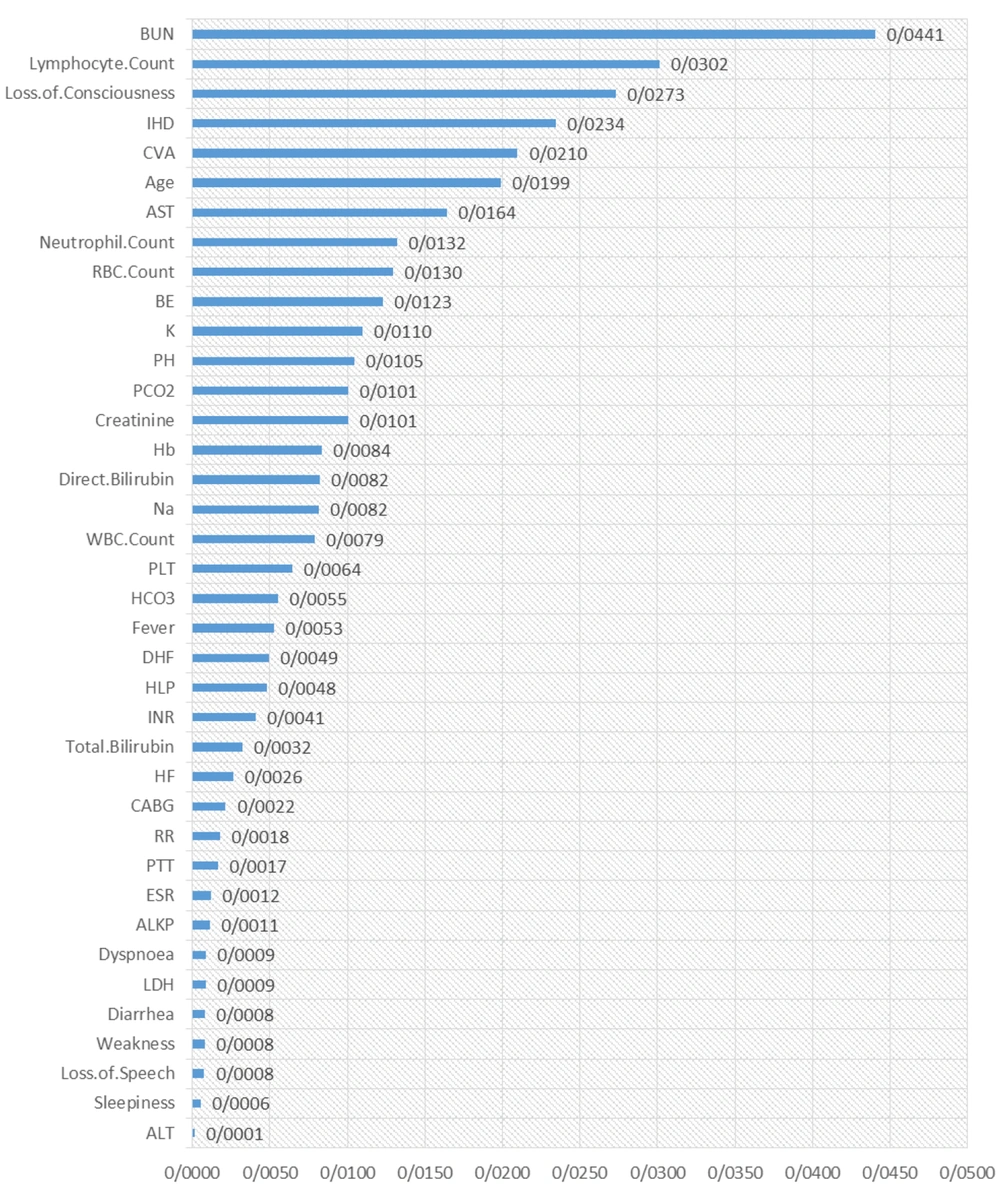
The results of the random survival forest are shown in Figures 1 and 2. The order of essential variables for admission as the starting time is shown in Figure 1, in which pH, WBC count, loss of consciousness, neutrophil count, BE, HCO3, age, BUN, O2 saturation, and lymphocyte count were at the top list. Moreover, some critical variables for symptom recognition as the starting time were BUN, lymphocyte count, loss of consciousness, IHD, Cerebrovascular accident (CVA), CVA, age, and AST. Other variables are shown in detail in Figure 2.
5. Discussion
In the current study, the mortality rate of COVID-19 was 30% and was significantly associated with critical disease intensity, fever, chills, loss of consciousness, IHD history, Parkinson's disease, invasive O2 therapy, and troponin levels. According to several studies, coronavirus infection, similar to some viral infections, may be associated with heart damage. A study of 400 patients admitted to Wuhan, China, found that about one-fifth of patients with COVID-19 had heart disease, which increases mortality (10). Severe and sudden inflammation of the heart muscle causes arrhythmia and impairs the heart's ability to pump blood efficiently. Therefore, patients with a history of cardiovascular disease and hypertension are at higher risk of death than normal individuals (11). Moreover, fatty plaques in the arteries of the heart of people with or without cardiovascular disease symptoms may become unstable due to fever and inflammation, leading to vascular occlusion and cardiovascular problems (12).
The current study declared that increased old age correlated with death in subjects suffering from COVID-19. In most studies, older age has been stated as a related predictor of fatality in SARS-CoV-2 and COVID-19 (13, 14). Opal in 2005 revealed that T-cell and B-cell function and the overproduction of interleukins become further acting by age, leading to a lack in control of viral replication and more extensive proinflammatory responses with harmful consequences (15).
We found that patient discharging was associated with higher O2 saturation, lymphocyte count, atrial blood pH, HCO3, and BE. Moreover, the higher mean of BS, BUN, total and direct bilirubin, neutrophil count, and sodium was associated with a higher discharge rate. Other essential studies confirm the mentioned factors in our study, and the results are somehow consistent (6, 14, 16). Li et al. in Wuhan in March 2020 presented that male gender, older subject, leukocytosis, cardiac injury, high blood glucose were associated with death in patients with severe COVID-19 (11). Similarly, in February 2020, Yang found that the increased risk of death of COVID-19 patients with pneumonia is considerable with older patients, duration from the onset of symptoms to ICU admission, ratio of PaO2 to FiO2, total bilirubin concentration, and lactate concentration (17).
The mean survival time with admission and symptom starting was approximately 12 and 21 days in the current research, respectively. Another study revealed the patient information based algorithm (PIBA) considered the death rate according to data of the subjects in Wuhan and then in other cities overall China. They calculated the predicted days from hospital admission to death was 13, and the mortality rate of COVID-19 varies from 0.75% to 3% and may decrease in the future (18). the study predicted the force of continuous exposure to coronavirus on the fatality rate gain and was used in Germany, China, France, United Kingdom, Iran, Italy, and Spain, for modeling. Regarding Iran, Italy, and Spain, the fatality rate will increase to 10% with an extra 3 - 10 days of exposure (19). However, for the dead time, the results are not consistent in different studies, and some have reported death up to 57 days after symptom onset (20).
Nevertheless, we found that cases have a higher probability of discharge when the clinical symptoms are diagnosed before the admission time. Finally, our results indicated that pH, WBC count, loss of consciousness, neutrophil count, BE, HCO3, age, BUN, O2 saturation, and lymphocyte count were at the top list of factors that affect the prognosis of the disease. Moreover, some critical variables for symptom recognition at the starting time were as follows: BUN, lymphocyte count, loss of consciousness, IHD, CVA, age, and AST. It is necessary to mention that most of the mentioned factors are the same in many studies but vary in importance. Garcia et al. reported creatinine, D-dimer, lactate, potassium, arterial pO2/FIO2 (P/F ratio), and alveolar-arterial gradient at admission and IHD as prognostic factors in patients with COVID-19 (20). Another study by Cummings et al. indicated that chronic pulmonary disease, chronic cardiovascular disease, older age, and elevated interleukin-6 and D-dimer levels at admission are the most substantial prognostic factors in patients with COVID-19 (21).
5.1. Conclusions
We hypothesize that the survival probability when symptom diagnosis is considered symptom diagnosis was considered the starting time is higher than that of admission time. In other words, cases had a higher probability of discharge when the clinical signs are diagnosed before than at the time of admission. Further, genetics, immune response, health care system, and other factors may affect the prognosis and change the most critical factors affecting the COVID-19 COVID_19 prognosis in different regions.