1. Background
Preeclampsia (PE) is a pregnancy disorder usually diagnosed with high blood pressure (systolic blood pressure ≥ 140 mmHg and diastolic blood pressure ≥ 90 mmHg) and proteinuria after the 20th week of pregnancy. Preeclampsia diagnosis is difficult in patients with chronic diseases, who suffer from high blood pressure or proteinuria at the same time. It can also lead to eclampsia, kidney and liver dysfunction, and coagulation system abnormalities (1-3).
Preeclampsia and fetal growth restriction (FGR) are the most important causes of perinatal death and complications in survivors, especially the increased risk of cardiovascular disease in mothers and newborns (4, 5). The risk of developing these complications increases in the presence of severe diseases, and may lead to preterm labor (PTL) before the 37th week of gestation (6, 7). Preeclampsia occurs in 1-8% of pregnant women on average, and its prevalence varies in different countries due to various risk factors. Overall, this disorder is the second leading cause of maternal mortality in the world (1, 8).
Some PE treatments include pregnancy termination, antihypertensive drugs, magnesium sulfate, beta-blockers, calcium channel blockers, and aspirin (9). Nowadays, aspirin is used as a treatment for PE. For this purpose, a study by Rolnik et al. showed that the use of aspirin in PE patients could be associated with a reduction in maternal and fetal complications (6, 10). Also, Wright and Nicolaides’s study showed that the use of aspirin prevented the progression of PE and improved the clinical condition of mother and fetus (11).
Thromboxane A2 (TXA2), which is responsible for vasoconstriction and platelet aggregation, increases in PE while prostacyclin, which mediates vasodilation and inhibits platelet aggregation, decreases, which are associated with increased risk of thrombosis in patients. Aspirin prevents this disorder by inhibiting the TXA2 secretion without affecting prostacyclin secretion. Therefore, the use of aspirin in patients can prevent thrombosis and placental abruption (1, 12-14).
2. Objectives
Due to the challenging results associated with PE treatment with aspirin and also very few studies about the effect of different doses of aspirin on the treatment process, we decided to evaluate the effect of different doses of aspirin on PE treatment.
3. Methods
This one-sided randomized clinical trial was done in Shahid Akbarabadi Medical Center during 2018-2019. The study was approved by the Ethics Committee of Iran University of Medical Sciences (IR.IUMS.FMD.REC.1398.133) and registered in the Iranian Clinical Trial Center (IRCT20190826044619N1). A simple convenience sampling method was utilized in this study. All pregnant women with a gestational age of 12-16 weeks, who had been referred to the hospital's prenatal clinic, were selected for the study. Patients were randomly assigned to the study groups (40 people in each group) based on block randomization with computer software. The first group received aspirin with the dose of 80 mg and the second group received the dose of 160 mg.
The inclusion criteria included pregnancy at 12 - 16 weeks of gestation, previous history of PE, diabetes, hypertension, and multipara. The exclusion criteria included the occurrence of problems and complications due to drug consumption (drug intolerance and gastric bleeding), subcutaneous bleeding, and platelet depletion.
At the first visit, demographic characteristics including age, body mass index (BMI), number of pregnancies, and systolic and diastolic blood pressures were recorded. Pregnant mothers were visited every two weeks until the 28th week of pregnancy, and then weekly until the end of the pregnancy. The drug consumption continued for 36 weeks and was then discontinued. Individuals, who developed blood pressure ≥ 140.90 mm Hg, proteinuria > +1 during a urine strip test, or proteinuria > 300 mg in 24-h urine during pregnancy, were monitored. Based on the PE severity, necessary measures were taken to terminate or continue the pregnancy.
If fetal intrauterine growth disorder was suspected, a Doppler ultrasound of the umbilical artery, midbrain, and venous duct was performed to confirm or rule out the fetal intrauterine growth restriction (IUGR). All subjects were monitored until the end of pregnancy. Pregnancy outcomes including PE, fetal IUGR, PTL, and delivery method were recorded. After birth, the Apgar score at first and fifth minutes and the weight of the infant were also recorded.
3.1. Statistical Analysis
After data collection using a questionnaire, the information was coded and entered into the computer. We used SPSS V.22 software for data analysis. An independent t test was performed to compare the continuous variables. The chi-square and the Fisher tests were also applied for qualitative variables. Logistic regression was also used to estimate the odds ratio of the effect. For each test, a P value < 0.05 was considered statistically significant.
4. Result
4.1. Demographical Information
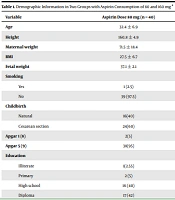
The final analysis was performed on 80 pregnant women in two groups (n = 40 in each group), with the aspirin dose of 80 mg (first group) and 160 mg (second group). The overall participants’ mean age was 32.3 ± 7.1, which was 32.4 ± 6.9 in the first group and 32.3 ± 7.4 in the second group; there was no significant difference between the two groups. In terms of education level, a significant difference was observed between the two groups (P value = 0.04). The causes of delivery such as the rupture of membranes (ROM), labor pain (LP), PTL, and other cases were higher in the second group than in the first group; this difference was not statistically significant (P value = 0.05). There was no significant difference between the two groups in terms of delivery type (normal vs. cesarean section), smoking, and type of occupation (Table 1).
| Variable | Aspirin Dose 80 mg (n = 40) | Aspirin Dose 160 mg (n = 40) | P Value b |
|---|---|---|---|
| Age | 32.4 ± 6.9 | 32.3 ± 7.4 | 0.82 |
| Height | 160.8 ± 4.9 | 164.07 ± 7.11 | 0.03 |
| Maternal weight | 71.5 ± 18.4 | 70.67 ± 12.9 | 0.15 |
| BMI | 27.5 ± 6.7 | 26.4 ± 4.7 | 0.18 |
| Fetal weight | 37.1 ± 2.1 | 37.05 ± 2.03 | 0.86 |
| Smoking | 0.30 | ||
| Yes | 1 (2.5) | 3 (7.5) | |
| No | 39 (97.5) | 37 (92.5) | |
| Childbirth | 0.36 | ||
| Natural | 16(40) | 20(50) | |
| Cesarean section | 24(60) | 20(50) | |
| Apgar 1 (8) | 2(5) | 0(0) | 0.1 |
| Apgar 5 (9) | 38(95) | 40(100) | 0.1 |
| Education | 0.04 | ||
| Illiterate | 1(2.55) | 0 (0) | |
| Primary | 2 (5) | 7(17.5) | |
| High school | 16 (40) | 6 (15) | |
| Diploma | 17 (42) | 24 (60) | |
| Academic | 4 (10) | 3 (7.5) | |
| Profession | 0.15 | ||
| Housewife | 38 (95) | 40 (100) | |
| Manual worker | 2 (5) | 0 (0) | |
| Cause of childbirth | 0.05 | ||
| LP | 4 (10) | 8 (20) | |
| ROM | 4 (10) | 8 (20) | |
| Pain | 3 (7.5) | 3 (7.5) | |
| PTL | 0 (0) | 6 (15) | |
| Fetal movement | 1(2.5) | 1 (2.5) | |
| Twin | 3 (7.5) | 0 (0) | |
| IUGR | 1 (2.5) | 1 (2.5) | |
| Heart arrest | 1 (2.5) | 0 (0) | |
| Preeclampsia | 4 (10) | 0 (0) | |
| RII | 3 (7.5) | 0 (0) | |
| Prolapse | 1 (2.5) | 0 (0) | |
| Other | 10 (25) | 9 (22.5) | |
| Combination c | 5 (12.5) | 4 (10) |
Abbreviations: BMI, body mass index; Lp, labor pain; ROM, rupture of membrane; PTL, preterm Labor; IUGR, intrauterine growth restriction; RII, repeat II.
a Values are expressed as No. (%).
bP value was calculated by chi-square or t test.
c Combination involved LP, ROM, pain, PTL, IUGR, and RII. Prolapse: the state when the umbilical cord comes out of the uterus, with or before the presenting part of the baby.
4.2. Pregnancy Complications
The complications of previous pregnancy, as well as those caused by aspirin consumption, were evaluated in the two groups. Based on the results, the complications of previous pregnancies such as PE, intrauterine fetal death (IUFD), and premature ROM (PROM) were higher in the first group; this difference was not statistically significant. Also, pregnancy complications due to aspirin consumption such as PE, hypertension, and diabetes were higher in the first group; this difference was not statistically significant, too (Table 2).
| Aspirin Dose 80 mg | Aspirin Dose 160 mg | OR (CI) | P Value b | |
|---|---|---|---|---|
| Present pregnancy complications | ||||
| Preeclampsia | 5 (12.5) | 1 (2.5) | 0.17 (0.003 - 1.7) | 0.08 |
| Hypertension | 4 (10) | 3 (7.5) | 0.7 (0.1 - 4.6) | 0.6 |
| Diabetes | 7 (17.5) | 2 (5) | 0.2 (0.2 - 1.4) | 0.07 |
| Combination | 5 (12.5) | 2 (5) | 0.4 (0.4 - 3.1) | 0.3 |
| Previous pregnancy complications | ||||
| Preeclampsia | 7 (17.5) | 5 (12.5) | 0.6 (0.1 - 2.7) | 0.5 |
| IUFD | 2 (5) | 0 | - | 0.15 |
| PROM | 3 (7.5) | 0 | - | 0.07 |
| Other | 21 (52.5) | 0 | - | < 0.001 |
Abbreviations: IUFD, intrauterine fetal death; PROM, premature rupture of membrane.
aValues are expressed as No. (%) unless otherwise indicated.
bP-value was calculated by chi-square or t test.
4.3. Fetus Complications
The previous history of fetal complications, as well as fetal complications due to aspirin use, was examined in both groups. The results showed that the incidence of IUGR and IUFD complications was higher in the first group, but no statistically significant difference was observed between them (Table 3).
| Aspirin Dose 80 mg | Aspirin Dose 160 mg | OR (CI) | P Value b | |
|---|---|---|---|---|
| Present Fetal Complications | ||||
| IUGR | 5 (12.5) | 3 (7.5) | 0.5 (0.8 - 3.1) | 0.4 |
| IUFD | 3 (7.5) | 0 (0) | - | 0.07 |
| Previous fetal complications | ||||
| IUGR | 2 (5) | 1 (2.5) | 0.4 (00.8 - 9.8) | 0.5 |
| IUFD | 1 (2.5) | 0(0) | - | 0.3 |
| Other | 0 (0) | 1 (2.5) | - | 0.3 |
Abbreviations: IUGR, intrauterine growth restriction; IUFD, intrauterine fetal death.
aValues are expressed as No. (%) unless otherwise indicated.
bP value was calculated by chi-square and t test.
4.4. Aspirin-derived Complications
Regarding the incidence of aspirin-related complications, the first group showed more wound infection, pain, and HIT, while the second group showed more hemorrhagic, gastrointestinal, and respiratory complications (P-value:0.01). Thus, there was a significant difference between the two groups in terms of aspirin-related complications (Table 4).
Abbreviation: HIT, heparin-induced thrombocytopenia.
aValues are expressed as No. (%).
bP value was calculated by chi-square or t test.
cCombination involved bleeding, gastrointestinal complications, respiratory complications, wound infection, pain, and PIT.
5. Discussion
Preeclampsia is one of the leading causes of maternal and perinatal mortality, with a prevalence of approximately 8% worldwide. It is characterized by placental abruption, high blood pressure, and proteinuria (15). High blood pressure can affect fetus development and lead to pregnancy disorders and complications such as premature birth and even fetal death. In addition, it has been shown that PE can be associated with some diseases and complications in the mother and fetus, including diabetes and cardiovascular diseases (16). Depending on the pathogenesis and progression of PE, it is divided into subgroups. Placental dysfunction can be one of the main causes of disease, which leads to disorders in the relationship between the mother and fetus.
Although many therapeutic strategies have been considered for PE treatment, due to the unknown pathogenesis of the disease, the main treatment has not been identified yet (17). Aspirin is one of the drugs, which is used to treat PE. Previous studies have shown challenging results regarding the effect of aspirin on PE treatment. However, very few studies have been performed on aspirin dose and its effect on PE treatment.
A study by Zhang et al. found that the incidence of maternal complications, including the recurrence of PE, diabetes, and hypertension, was higher in patients receiving aspirin than in controls. These results indicated that aspirin consumption in patients could not play a protective role against PE recurrence (18). Another study by Ling et al. reported a reduced incidence of maternal complications due to aspirin consumption; the cited complications included PE, diabetes, and heart disease, which were statistically significant in PE patients (19).
The present study showed that the incidence of maternal complications such as diabetes, hypertension, and PE was higher in the group receiving 80 mg aspirin than in the group receiving 160 mg, but this difference was not statistically significant. Although the incidence of maternal complications decreased with increasing aspirin dosage, the lack of significant correlation could be due to the low sample size, which needs to be evaluated in a larger number of patients in future studies.
Fetal complications are another factor that can be prevented by aspirin prescription in PE patients. A study by Abdi et al. declared that the use of aspirin reduced the incidence of complications such as IUGR (20). Vanda et al. showed that the use of aspirin (80 mg) reduced maternal and fetal complications in PE mothers (21). The present study also suggested that increasing aspirin dosage would prevent complications such as IUGR and IUFD.
A study by Hastie et al. also reported bleeding as a result of aspirin consumption during pregnancy (22). This study also showed that the aspirin side effects, including bleeding, were higher at the dose of 160 mg, which was statistically significant.
5.1. Conclusions
It can be concluded that increasing aspirin dosage reduces the incidence of maternal and fetal complications in PE mothers. It should be noted that bleeding is one of the side effects of aspirin. The monitoring of patients during treatment procedures should be considered to prevent possible bleeding.