1. Background
Non-alcoholic fatty liver disease (NAFLD) is defined as excessive accumulation of liver fat in the absence of alcohol consumption in more than 5% to 10% of liver weight (1). The prevalence of this disease is estimated at 20 - 35% in Western countries and 19 - 32% in Asia (2). Hepatic steatosis often leads to the inflammation of the liver or non-alcoholic steatohepatitis (NASH) and, if left untreated, can lead to liver fibrosis and cirrhosis (1).
After menopause, the prevalence of NAFLD increases in women compared to men due to hormonal changes, weight gain, and abdominal obesity. Obesity, as chronic low-grade inflammation, is the main cause of fatty liver, which leads to increased risk factors such as insulin resistance, dyslipidemia, the release of fatty acids, and activation of inflammatory pathways in the liver, leading to liver damage (1, 3). Cytokines are proteins that in the two groups of T helper 1 and T helper 2 cells lead to the secretion of proinflammatory cytokines including IL18, TNFα, IL6, and anti-inflammatory factors such as IL10 (3, 4). In normal circumstances, there is no or very little production of these inflammatory factors in the liver, but factors such as fat accumulation cause their production (5).
As known, IL18 is a proinflammatory cytokine belonging to the IL-1 family of cytokines. It is secreted in many cells, including macrophages, Kupffer cells, and adipose tissue. Serum levels of this cytokine are associated with metabolic syndrome, obesity, especially visceral obesity, and insulin resistance. Some studies have shown an increase in plasma levels of IL18 in patients with fatty liver and reported that IL18 can affect insulin sensitivity and lipid metabolism by disrupting insulin and lipid signaling pathways (6-8). Flisiak-Jackiewicz et al. (6) reported that IL18 concentration was higher in patients with NAFLD than in the control group and showed an association between IL18 and hepatic steatosis. However, Tapan et al. (9) reported that IL18 was not involved in the pathogenesis of hepatic steatosis and NASH. Besides, IL10 is an anti-inflammatory cytokine that plays an important role in regulating the immune system and improving liver cell damage. It is produced by macrophages, lymphocytes, and Kupffer cells and reduces inflammation by some mechanisms, including inhibiting the production of proinflammatory cytokines such as IL1, IL6, and TNFα (10, 11). Also, IL10 has been reported to be involved in NAFLD and insulin resistance, and by affecting peripheral glucose metabolism, it reduces insulin resistance (11, 12).
Decreased levels of IL10 have been observed with increasing age. In adults, IL10 levels decrease in diseases such as cardiovascular disease, obesity, type 2 diabetes, and NAFLD (4, 10). Paredes-Turrubiarte et al. (13), in a study of 102 obese patients with fatty liver, showed a decrease in IL10 levels according to the severity of NAFLD. However, there is disagreement about the role of IL10 in hepatic steatosis due to a high-fat diet and insulin resistance, and it has been reported that IL10 does not prevent insulin resistance (11).
Evidence suggests that exercise is a non-pharmacological method for reducing systemic inflammation and treating non-alcoholic fatty liver disease. Performing both aerobic and resistance training exercises can have significant benefits in creating physical fitness and health-related factors in elderly, obese, or sick people (10, 14). However, the effect of training on inflammatory markers has been reported to be contradictory. Lee and Chung (15) reported a decrease in IL18 levels in rats after 12 weeks of moderate treadmill activity, while Khakroo Abkenar et al. (16) did not observe a significant effect on IL18 after three months of moderate-intensity acute aerobic training. Pereira et al. (17) also showed an increase in IL10 levels in 451 elderly women after three months of regular exercise compared to the inactive group; nevertheless, in the study conducted by Pereira et al. (17) in 2013, resistance training did not alter serum IL10 levels in women with metabolic syndrome.
2. Objectives
Due to the contradictory results and lack of sufficient evidence on the effectiveness of combined exercise on inflammatory markers IL18 and IL10 in patients with fatty liver, this study aimed to investigate the response of inflammatory biomarkers to 10 weeks of aerobic resistance training in inactive postmenopausal women with non-alcoholic fatty liver.
3. Methods
3.1. Subjects
This quasi-experimental study was conducted in Mahshahr, Iran, in 2019. From among patients of the health center, 24 postmenopausal women were purposefully selected and randomly divided into experimental (n = 12) or control (n = 12) groups. The sample size was obtained by the following equation:
The inclusion criteria encompassed women aged 50 to 68 years, with a normal menstrual interruption for at least one year and fatty liver disease, and the exclusion criteria comprised a history of liver disease, smoking and alcohol use, hormone therapy, and regular exercise in the past year. Personal information, sports history, menopausal status, and their medications (metformin 500 and atorvastatin 20) were determined by a questionnaire based on PARQ (18).
In all participants, the type and the amount of the used drug were the same. Matching the participants’ nutrition with the food recall questionnaire was performed. Also, the control group did not participate in any training activities. This study was approved by the Research Ethics Committee of the University of Isfahan with code IR.UI.REC.1398.011. Being informed of the objectives of the research and the confidentiality of the data, the participants signed the consent form and were free to leave the study if they wished. Due to the withdrawal of two participants in the training group, the information of 22 participants was ultimately evaluated.
3.2. Measurements
Before and 48 hours after the end of the study, abdominal ultrasound was performed by a physician using a GE Voluson 730 device (made in the USA). Due to increased echogenicity of the liver, the disease was diagnosed as grades 1, 2, and 3 (3). To examine biochemical variables, 10 cc of blood was taken from the arm vein of each subject in the fasting state. Then, AST, TG, and fasting glucose were evaluated using Pars Azmoon kits, and serum levels of interleukin 18 and interleukin 10 were assessed by ELISA method using the German Zelbio kit. Insulin was measured using German Roche kit by Electrochemiluminescence. The participants’ weight was measured with a German digital scale (Beurer), and their waist circumference (abdominal obesity index) was measured in the narrowest area. The insulin resistance index was calculated by the following formula (19):
3.3. Training Protocol
The training protocol consisted of 10 weeks of aerobic resistance training performed three times a week. After warming up for 10 minutes with walking, stretching, and using an ergometer bicycle, the participants began aerobic training on a treadmill with an intensity of 50% of maximum heart rate (HRmax) and increased it to 75% of maximum heart rate by the last two weeks. Following each session, resistance training was performed for about 45 minutes with bodybuilding devices, including axillary pull-ups, forearm pull-ups, back pull-ups, chest presses, and leg presses. The participants started resistance training at 50% intensity of one repetition maximum (1RM) and two sets of 10 repetitions, and increased it to 75% of 1RM and three sets of 10 repetitions in the last two weeks (Table 1) (2). At the end of each session, the body was cooled by walking and stretching. Brzycki formula was used to calculate one repetition maximum (1RM) (20):
MHR, maximum heart rate; 1RM, one repetition maximum.
| Week | Aerobic Training | Resistance Training | ||
|---|---|---|---|---|
| Intensity (MHR), % | Period, min | 1RM, % | Set | |
| 1 - 2 | 50 | 15 - 20 | 50 | 2 |
| 3 - 4 | 60 | 25 | 60 | 2 |
| 5 - 6 | 65 | 30 | 60 | 3 |
| 7 - 8 | 70 | 30 | 70 | 3 |
| 9 - 10 | 75 | 30 | 75 | 3 |
3.4. Statistical Analysis
All data were analyzed using SPSS version 22 software. The Kolmogorov-Smirnov statistical test was used to check the normality of the data. Also, to initially compare the data of the two groups, the independent samples t-test for normal variables and Mann-Whitney U-test for non-normal variables were used. Then, the paired samples t-test for normal variables and the Wilcoxon test for non-normal variables were used to determine intragroup changes. Besides, the comparison of intergroup changes in the posttest stage was performed by analysis of covariance (ANCOVA). The relationships of IL18 and IL10 with each other and with other studied variables were determined by the Spearman correlation test. A P < 0.05 was considered the significance level.
4. Results
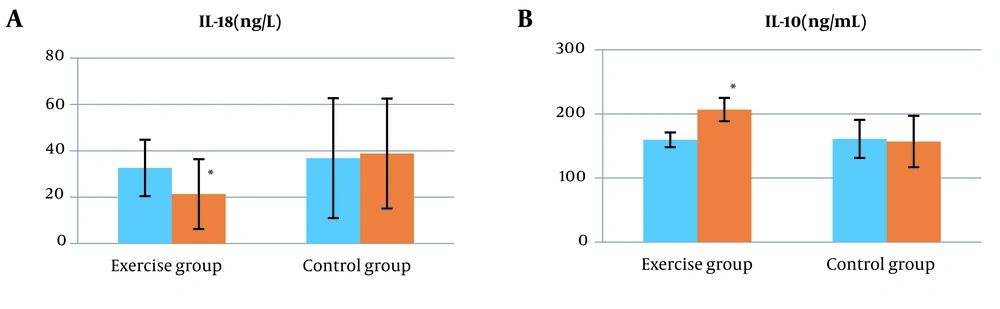
Basic information of the participants is presented in Table 2. Based on the results obtained in the pretest stage, there was no significant difference between the two groups (P > 0.05). Intragroup findings by paired samples’ t-test and Wilcoxon test showed that 10 weeks of aerobic resistance training significantly reduced IL18, body weight, WC, liver enzymes ALT and AST, fasting glucose, triglyceride, insulin resistance index, and liver fat and significantly increased IL10 in the training group compared to the control group (P < 0.05). The intragroup decrease in fasting insulin levels was not significant in the training group compared to the control group (P > 0.05) (Table 3 and Figure 1).
| Variable | Pretest | P |
|---|---|---|
| Age, y | 0.941 | |
| Training | 56.10 ± 3.21 | |
| Control | 56.25 ± 5.62 | |
| Weight, kg | 0.109 | |
| Training | 78.39 ± 8.86 | |
| Control | 84.12 ± 7.19 | |
| Waist circumference, cm | 0.301 | |
| Training | 97.10 ± 10.40 | |
| Control | 101.16 ± 7.52 | |
| Liver fat (grades 1, 2, 3) | 0.887 | |
| Training | 1.80 ± 0.78 | |
| Control | 1.83 ± 0.71 | |
| IL18, ng/L | 0.640 | |
| Training | 32.60 ± 12.17 | |
| Control | 36.83 ± 25.86 | |
| IL10, ng/mL | 0.575 | |
| Training | 159.60 ± 11.49 | |
| Control | 161.00 ± 29.70 | |
| ALT, IU/L | 0.259 | |
| Training | 23.90 ± 7.92 | |
| Control | 20.00 ± 6.17 | |
| AST, IU/L | 0.114 | |
| Training | 25.70 ± 7.68 | |
| Control | 21.25 ± 4.84 | |
| TG, mg/dL | 0.851 | |
| Training | 195.10 ± 22.88 | |
| Control | 192.83 ± 31.26 | |
| Fasting insulin, micIU/mL | 0.566 | |
| Training | 14.92 ± 8.38 | |
| Control | 12.76 ± 8.78 | |
| Fasting glucose, mg/dL | 0.391 | |
| Training | 166.00 ± 89.34 | |
| Control | 136.75 ± 64.44 | |
| HOMA-IR | 0.322 | |
| Training | 6.03 ± 4.96 | |
| Control | 4.10 ± 2.84 |
aValuse are expressed as mean ± SD.
bIntergroup results in the pretest with independent t-test and Mann-Whitney U-test.
| Variable | Pretest | Posttest | P Intragroup | P Between Group |
|---|---|---|---|---|
| Weight, kg | 0.000** | |||
| Training | 78.39 ± 8.86 | 76.12 ± 9.32 | 0.000* | |
| Control | 84.12 ± 7.19 | 84.34 ± 7.32 | 0.273 | |
| Waist circumference, cm | 0.000** | |||
| Training | 97.10 ± 10.40 | 91.50 ± 10.12 | 0.000* | |
| Control | 101.16 ± 7.52 | 101.00 ± 7.43 | 0.586 | |
| ALT, IU/L | 0.073 | |||
| Training | 23.90 ± 7.92 | 19.90 ± 7.44 | 0.004* | |
| Control | 20.00 ± 6.17 | 19.58 ± 5.75 | 0.560 | |
| AST, IU/L | 0.046** | |||
| Training | 25.70 ± 7.68 | 20.00 ± 5.09 | 0.016* | |
| Control | 21.25 ± 4.84 | 22.16 ± 6.76 | 0.528 | |
| TG, mg/dL | 0.000** | |||
| Training | 195.10 ± 22.88 | 161.00 ± 23.68 | 0.000* | |
| Control | 192.83 ± 31.26 | 193.91 ± 32.20 | 0.586 | |
| Fasting insulin, micIU/mL | 0.114 | |||
| Training | 14.92 ± 8.38 | 11.35 ± 6.51 | 0.143 | |
| Control | 12.76 ± 8.78 | 13.16 ± 6.95 | 0.710 | |
| Fasting glucose, mg/dL | 0.003** | |||
| Training | 166.00 ± 89.34 | 133.5 ± 62.82 | 0.005* | |
| Control | 136.75 ± 64.44 | 135.33 ± 63.09 | 0.408 | |
| HOMA-IR | 0.011** | |||
| Training | 6.03 ± 4.96 | 3.57 ± 3.26 | 0.028* | |
| Control | 4.10 ± 2.84 | 4.24 ± 2.92 | 0.432 | |
| Liver fat | 0.000** | |||
| Training | 1.80 ± 0.78 | 0.70 ± 0.67 | 0.002* | |
| Control | 1.83 ± 0.71 | 1.91 ± 0.79 | 0.564 |
aValuse are expressed as mean ± SD.
b*, Significant difference within the group; **, significant difference between the groups; significance level (P < 0.05).
Intergroup findings by ANCOVA after 10 weeks of combined training showed that IL18, body weight, WC, AST, triglyceride, fasting glucose, insulin resistance index, and liver fat based on ultrasound decreased significantly in the training group compared to the control group (P < 0.05) (Table 3 and Figure 1). Besides, ALT and fasting insulin did not show a significant difference between the groups (P > 0.05) (Table 3). Also, after 10 weeks of training, a significant increase in IL10 levels was observed (P < 0.05) (Figure 1).
Using the Spearman correlation test, a significant positive relationship between IL18 and WC, ALT, and HOMA-IR was determined. Also, IL10 had a significant negative relationship with body weight, WC, triglyceride, HOMA-IR, and liver fat. Besides, a significant inverse relationship was observed between IL18 and IL10 (P < 0.05) (Table 4).
| Variable | IL18, ng/L | IL10, ng/L | Weight, kg | Waist Circumference, cm | ALT, IU/L | AST, IU/L | TG, mg/dL | Fasting Insulin, micIU/mL | Fasting Glucose, mg/dL | HOMA-IR | Liver Fat (Grade 1, 2, 3) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Il18, ng/L | |||||||||||
| r | -0.472 | 0.093 | 0.440 | 0.557 | 0.392 | 0.135 | 0.365 | 0.396 | 0.643 | 0.374 | |
| P | 0.027* | 0.679 | 0.040* | 0.005* | 0.072 | 0.548 | 0.095 | 0.068 | 0.001* | 0.087 | |
| Il10, ng/mL | |||||||||||
| r | -0.472 | -0.443 | -0.563 | -0.343 | -0.185 | -0.428 | -0182 | 0.388 | -0.435 | -0.636 | |
| P | 0.027* | 0.039* | 0.006* | 0.118 | 0.410 | 0.047* | 0.418 | 0.074 | 0.043* | 0.001* |
aSpearman correlation coefficient values; *, level of significance (P < 0.05).
5. Discussion
The results of the present study showed a significant decrease in IL18, a significant increase in IL10, and improvement in the fatty liver after 10 weeks of aerobic resistance training in postmenopausal women with NAFLD.
A significant decrease in IL18 could indicate an improvement in immune function and a reduction in inflammation in response to exercise training. Consistent with these results, Troseid et al. (21) showed a decrease in IL18 after 12 weeks of aerobic and strength training in people with metabolic syndrome, and reported that the protective effect of exercise could be due to reduced inflammation. However, Zaidi et al. (22) did not observe a significant change in IL18 after 12 months of combined training in patients aged 41 to 81 years, which can be due to the lack of weight loss and insufficient intensity of training.
Some studies have identified adipose tissue depletion as an important factor in lowering serum levels of this cytokine, because adipose tissue is known as one of the most important sources of IL18 production (21). In this study, a significant reduction in body weight and waist circumference was observed after training, and IL18 levels had a significant positive relationship with waist circumference, which agrees with the results of Yamaoka-Tojo et al.’s study (7) that showed weight loss reduced il18 and reported an interaction between waist circumference and il18 levels. Performing exercise has been shown to positively change body composition and increase lipolysis in abdominal adipose tissue (23), and reducing the production of proinflammatory cytokines/chemokines and inhibiting the penetration of immune cells into adipose tissue is one of the effective anti-inflammatory mechanisms (24). In addition, cytokines are involved in regulating skeletal muscle function. Because resistance training was part of the exercise training protocol in this study, improvement in muscle function appears to be associated with IL18 changes (21). Also, based on the present results, after 10 weeks of training, a significant decrease in fasting glucose and insulin resistance was observed, and fasting insulin levels decreased. Insulin resistance is one of the most important parameters to change during menopause, which causes the accumulation of TG and increases the risk of NAFLD (1). Some studies have reported that IL18 levels are implicated in insulin resistance (22). Our results also showed a high correlation between IL18 and insulin resistance. The decrease in IL-18 after exercise training appears to be in part due to the beneficial effects of exercise on insulin resistance.
Also, in this study, a significant reduction in triglyceride and liver fat was observed based on ultrasound evidence, which could be due to significant changes in body weight and abdominal obesity after exercise training (19); however, there was no significant relationship between IL18 and TG, but a decrease in IL18 and insulin resistance after 10 weeks of training could be sufficient to reduce inflammation and improve fatty liver, which is consistent with the results of Borai et al.’s study (3) who reported that when IL18 is combined with the insulin resistance index, it is associated with varying degrees of non-alcoholic fatty liver disease.
Another important finding of this study was a significant increase in IL10 levels in the training group compared to the control group. Consistent with our results, Batista et al. (25) after 8 - 10 weeks of endurance training in sick rats and Eizadi et al. (10), after six and 12 weeks of aerobic training in obese women, reported a significant increase in IL10 levels (10, 25). However, Ghafourian et al. (26) and Conroy et al. (27) did not observe a significant increase in IL10 levels after training, which is probably due to the selection of healthy subjects and normal metabolic and inflammatory conditions.
One of the causes of increased IL10 levels after exercise training is weight loss and reduced body fat as a result of increased fat oxidation. In this study, IL10 had a significant inverse correlation with body weight and waist circumference, which shows that weight loss and abdominal obesity due to adaptation to exercise training can be the reasons for increasing IL10, which is consistent with the results of Eizadi et al.’s study (10). It has also been reported that the balance between pro-inflammatory and anti-inflammatory active cytokines plays an important role in NAFLD (4). In our study, a significant inverse correlation between IL10 and IL18 was observed. Increasing IL10 by regulating the production of proinflammatory cytokines may be effective in inhibiting IL18. Also, in the present study, IL10 had a significant negative relationship with triglyceride, insulin resistance, and liver fat. It seems that decreased inflammation and fat in the liver appear in response to aerobic resistance exercise through increased IL10. This is in line with the results of Hong et al. (12), who showed that IL10 increases insulin sensitivity and disagrees with Asrih and Jornayvaz (11), who reported that IL10 does not prevent insulin resistance.
Another finding of the present study was a decrease in liver enzymes AST and ALT after 10 weeks of training. Recently, ALT has been shown to be involved in the development of insulin resistance and TG accumulation in the liver along with oxidative stress and inflammation (28). In the present study, a significant positive relationship was observed between IL18 and ALT. Reducing these factors after exercise training indicates a reduction in inflammation and improvement in liver fat, which is consistent with the findings of Flisiak-Jackiewicz et al. (6) who showed an association between IL18 and ALT and liver damage in NAFLD patients. In our study, an inverse relationship was found between IL10 and liver enzymes, but it was not significant, which is consistent with the results of Bruno et al.’s study (29).
5.1. Limitations
One of the limitations of this study was the lack of precise nutritional control of the subjects and the lack of examination of other inflammatory factors. While emphasizing combined training in patients with NAFLD, it is recommended to follow such a protocol by observing the nutritional diet and examining other inflammatory factors.
5.2. Conclusions
This study revealed that aerobic resistance training can reduce IL18 levels by reducing obesity, especially abdominal obesity; it also can play an important role in improving non-alcoholic fatty liver by increasing IL10 and insulin sensitivity, as well as improving liver enzymes.