1. Background
Renal colic is a common complaint among patients referring to emergency departments (EDs) (1% of presentations to EDs) (1), and it is seen in 5 - 15% of subjects’ lifetime (2). The movement of renal stone in the urinary tract is a common cause of severe groin pain. Both non-steroidal anti-inflammatory drugs (NSAIDs) and opioids are gold standards for relieving pain with acute renal colic (3, 4). Although opioids are potent and cheap, but they have some adverse events (AEs) such as nausea, vomiting, constipation, respiration depression (in larger doses), drowsiness, and hypotension (5). Compare with NSAIDS, opioid has fewer complications such as drowsiness, vomiting, and nausea; but it was demonstrated they can develop AEs such as nephropathy and platelet dysfunction (6). Concerning various AEs of opioids and NSAIDs, discovering new drugs with adequate analgesic properties and fewer AEs is essential (7).
Ketamine is an arylcyclohexylaminethat acts mainly on glutamate binding sites, N-Methyl-D-Aspartate receptors (NMDA), and non-NMDA receptors cause psych sensory effects, amnesia analgesia, and neuroprotection (8). Ketamine is used as a procedure not requiring any musculoskeletal relaxation or in short surgeries. This drug can be prescribed for all groups of patients except in exceptional cases such as patients with severe hypertension, brain traumas, cardiovascular diseases, and heart attack (9, 10). Ketamine is administered intravenously and/or intramuscularly (11), but it can also be administered intranasally with few effects on the heart (12). As far as the researchers investigated, there is not sufficient clinical studies on the efficacy of intranasal Ketamine on renal colic.
2. Objectives
This study aimed to evaluate the clinical efficacy of intranasal Ketamine in renal colic and compare it with traditional treatment methods [intravenous NSAIDs (Ketorolac)].
3. Methods
3.1. Study Design
The current survey is a single-center, randomized phase III clinical trial to compare the effect of intranasal Ketamine versus intravenous Ketorolac for pain control in the renal colic patients in Imam Khomeini Hospital of Ahvaz, Iran from November 2019 to May 2020. The study protocol was approved by the Medical Ethics Committee of Jundishapur Ahvaz University (reference number: IR. AJUMS.U-97023) and it was performed as a phase III clinical trial (code: IRCT20180526039846N1).
3.2. Participants
Based on clinical and para-clinical findings [ultrasound and computed tomography (CT) scan], the patients were identified by emergency medicine specialists. All participants signed a written informed consent.
3.3. Inclusion Criteria
The inclusion criteria were: (1) patients referred to ED with acute renal pain (severity more than five based on a visual analog scale [VAS]) based on the radiological examinations and clinical history; (2) aged over 18 years; and (3) not received analgesic drugs for at least six hours.
3.4. Exclusion Criteria
Patients with a history of allergy to ketamine or ketorolac (toradol), aspirin or ibuprofen, contraindications to ketorolac or ketamine, unstable vital signs, fluctuations in alertness, underlying diseases such as liver cirrhosis (chronic alcoholism), recent gastrointestinal bleeding, and perforation in the last five days or renal failure, previous renal surgery, hypertension, history of any type of cardiac diseases, abdominal tenderness suspected of peritonitis, chronic respiratory diseases, nasal obstruction, brain tumors, acute head traumas, history of seizure, any type of metabolic diseases, history of peptic ulcer, glaucoma, history of drug addiction, history of psychological disorders, malignancy, pregnancy and lactation, unconfirmed stone-induced renal colic, and body temperature over 38°C were excluded.
3.5. Intervention
The control group received Ketorolac 30 mg/kg (in bolus form) and placebo intranasally. In the intervention group, Ketamine 1 mg/kg was prescribed intranasally and placebo intravenously. Patients with a VAS score ≥ 3 received 1 μg/kg of IV fentanyl as rescue analgesia. In patients suffering from dehydration or sepsis, 10 mL/kg liquid therapy was used. During the treatment period, all patients were under the supervision of emergency medicine specialists. Also, patients with incomplete data were excluded.
3.6. Outcomes Assessment
The pain intensity was a 10-cm horizontal line. This line was written with ‘pain with the most intensity’ on the right with a score of 10 and ‘no pain’ on the left with a score of zero (13). The primary outcome was assessed for pain reduction before administration, 5, 15, 30, and 45 minutes after administration. For this evaluation, relief pain ≤ 30 based on VAS score was considered significant. After 30 minutes of the drug administration, no significant reduction in pain was observed (∆VAS ≥ 3); the Fentanyl 1 µg/kg with 5-minute interval time was prescribed until the intensity of pain was reduced. The secondary outcome was assessed according to the time of staying in an emergency room (between 1 - 2 hours), the value of financial of treatment, the need for lifesaving therapy (used for patients with VAS ≥ 5 after 60 minutes), side effects after 60 minutes, and measuring satisfaction based on 5-point Likert scale (0 = poor to 4 = excellent) 60 minutes after administration. Vital signs of the patients and AEs after treatment, such as dizziness, rash, headache, tachycardia, breath distress, blurred vision, and vomiting were recorded.
3.7. Statistical Analysis
To compare qualitative variables and determine the normal distribution of quantitative parameters, chi-square test and the Kolmogorov-Smirnov test were used, respectively. T-test and Mann-Whitney test were used for variables with normal and without distribution, respectively. To evaluate the differences between the groups in different periods, repeated measures ANOVA was used. A P-value < 0.05 was considered as significant. All data were analyzed by SPSS software (V24).
4. Results
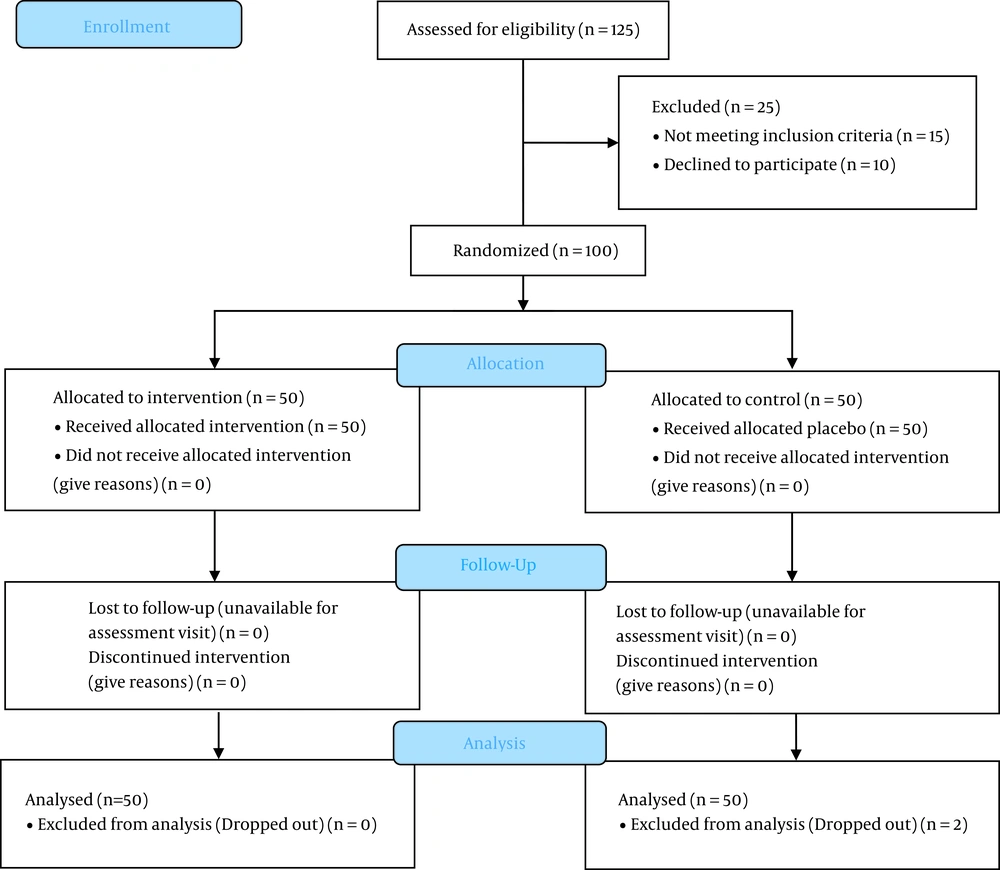
In this study, a total of 100 patients were randomly divided into two groups (intranasal Ketamine and intravenous Ketorolac) (Figure 1). The patients' mean age was 34.39 ± 5.60 years, and 71 (71%) were male (Table 1). In patients who did not respond to treatment after 30 minutes, fentanyl 1 µg/kg with 5-minute interval time was prescribed until the pain reduced; in this regard, no patients were excluded from the study.
| Variables | Intranasal Ketamine (n = 50) | Intravenous Ketorolac (n = 50) | P-Value |
|---|---|---|---|
| Age (y) | 33.66 ± 7.61 | 34.84 ± 6.67 | 0.412 |
| Sex (male) | 35 (70) | 36 (72) | 0.826 |
| Pain (VAS score) | |||
| Before intervention | 9.62 ± 0.53 | 9.5 ± 0.54 | 0.239 |
| After 5 min | 5 ± 0.4 | 8.62 ± 0.49 | < 0.001 |
| After 15 min | 1.6 ± 0.49 | 6.8 ± 0.53 | < 0.001 |
| After 30 min | 0.6 ± 0.57 | 3.68 ± 0.47 | < 0.001 |
| After 45 min | 0.6 ± 0.57 | 2.06 ± 0.72 | < 0.001 |
| After 60 min | 0.5 ± 0.5 | 1.96 ± 0.69 | < 0.001 |
| AEs | 0 | 4 (8) | 0.117 |
| Fentanyl administration | 0 | 11 (22) | 0.001 |
| Hospitalization duration (min) | 89.3 ± 10.35 | 101.04 ± 15.25 | < 0.001 |
| Patients' satisfaction | 3.56 ± 0.35 | 1.82 ± 0.98 | < 0.001 |
Abbreviations: VAS, visual analog scale; AEs, adverse events.
a Values are expressed as mean ± SD and No. (%).
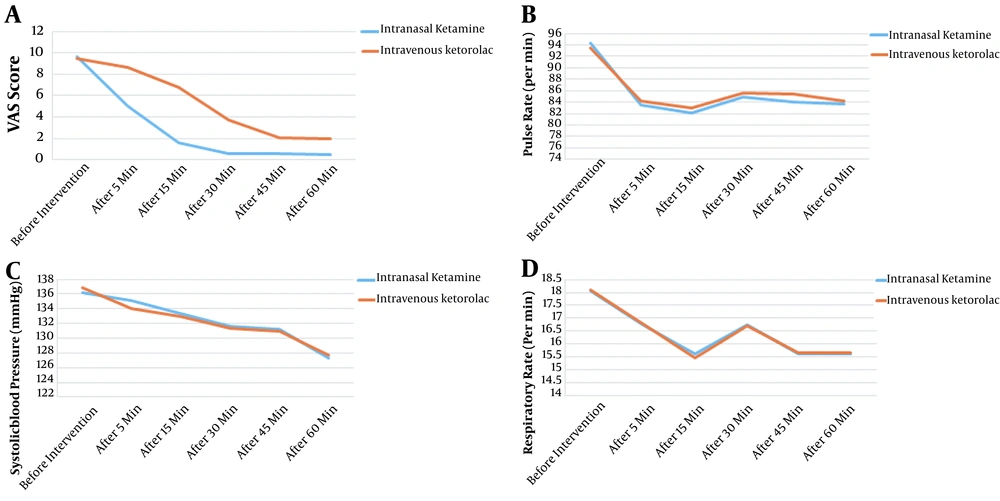
Before the intervention, there was no significant difference between the two groups in terms of the studied variables, including VAS score and hemodynamic parameters (P > 0.05). The mean VAS score in the intranasal Ketamine and control groups after the first 5 minutes (5 ± 2.26 vs. 8.62 ± 0.49, respectively; P < 0.001) and 60 minutes (P < 0.001) was lower in the intervention group as compared to the control. A substantial decreasing pattern in pain level was found in the two groups from the start to minutes 5 and 60 (P < 0.001) (Figure 2A). Furthermore, pulse rate did not differ between the two groups at any time (P > 0.05), and the repeated measures ANOVA test did not show differences between the two groups in different periods (F = 0.985, P = 0.323) (Figure 2B). The use of fentanyl to control AEs was more frequent in the control group (22 vs. 0%) (P = 0.001) (Table 1). The frequency of AEs was the same between the two groups (P = 0.117).
Our results showed that hospitalization duration in ED was remarkably lower in the intranasal Ketamine group (89.3 ± 10.35 min) as compared to the intravenous Ketorolac group (101.04 ± 15.25 min) (P < 0.001). No significant differences were seen in terms of blood pressure (F = 0.006, P = 0.938) and respiratory rate (F = 0.002, P = 0.966) between the two groups (Figure 2C and D). Finally, patients' satisfaction was significantly higher in intranasal Ketamine group compared to the control (3.56 ± 0.35 vs. 1.82 ± 0.98, respectively; P < 0.001).
5. Discussion
Pain management is vital in patients with renal stones, and most medications used for this purpose are intramuscular and intravenous or oral opioids and NSAIDs. Ketamine, as an opium alternative, is an analgesic drug in relieving acute pain with different administration methods such as intranasal (14). The bioavailability of intranasal Ketamine is 45%, the peak volume of plasma was seen in less than 30 minutes, and the terminal half-life is around 2 hours (15).
According to our results, renal colic-induced pain was controlled better in cases treated with intranasal ketamine compared to intravenous ketorolac in the first 5 minutes after drug administration. There were no significant differences in rate of complications and hemodynamic changes between the intervention and control groups. On the other hand, our findings showed that the additional analgesia administration was significantly lower in the intranasal ketamine group. Finally, intranasal ketamine increased patient satisfaction. This is inconsistent with the recent findings that intranasal ketamine can be helpful when accompanied by other medications (16). One explanation for this difference can be the administration of different drugs in control group. Pouraghaei et al. compared the effect of intranasal ketamine versus intravenous morphine, and showed that intranasal ketamine is more effective and it has lower AEs (17). This is in line with the results of Farnia et al., which indicated that intranasal ketamine could help alleviate pain compared to intravenous morphine (18).
Carr et al. showed that blood level of ketamine in intranasal administration can reach a detectable level after two minutes, with a median duration of nearly 30 minutes (19). Moreover, other studies showed that the effect of intranasal ketamine in reducing pain would last for three hours (20). In our study, the pain was relieved 5 and 60 minutes post-administration, whereas in the study by Shrestha et al. the pain score decreased 15 minutes after administration (21).
Likewise, intranasal ketamine was observed by Yeaman et al. within 30 minutes, in which the pain severity was lowered by 2.4 units (22). Furthermore, Andolfatto et al. (23) showed that intranasal Ketamine can minimize the pain score depending on VAS score by at least 1.3 units after 30 minutes, which is inconsistent with the present survey. In contrast, no significant differences for reliving pain scores were observed in the same study (24).
After analyzing for AEs, our data demonstrated that dizziness and nausea were the most common AEs among patients, which is consistent with the studies by Shrestha et al. and Andolfatto et al. Additionally, their survey revealed intranasal ketamine could be useful in patients with acute injury (21, 23). The short follow-up period and a small sample size were the main limitations of the current investigation.
5.1. Conclusions
This findings of current study revealed the positive benefits of intranasal ketamine compared to standard treatment in managing pain due to renal colic. This substantial therapy can contribute to dramatically increasing short-term pain regulation with reduced AEs and enhanced patient satisfaction.